Growth Factor Enhancement to Improve Implant Outcomes
Advantages of using PRP and PRF therapies for implant patients
Jamie Oshidar, DMD
Private Practice
Roselle Park, New Jersey
Lecturer
ImpactImplantSeminars.com
To achieve successful dental implant placement, one of the essential conditions is the presence of sufficient residual bone at the site. Much research has been done to improve the efficiency of bone grafting. As explained and demonstrated in the case presentation, one method of ameliorating bone-graft healing involves the use of growth factor enhancement.
Growth Factors
Growth factors are bioactive proteins that have a role in controlling biological processes, such as cell growth, proliferation, and differentiation; they also can facilitate hard- and soft-tissue repair and regeneration.1 After binding to specific cell surface receptors, growth factors can then target cells in a number of recognized ways or modes and orchestrate the complex sequence of cell migration, division, differentiation, and protein expression during wound healing.
There are eight major classes of growth factors2 that are expressed in varying levels by the cells involved with healing; the effects of each growth factor are regulated through a complex system of feedback loops. Advances in the areas of cellular and molecular biology have allowed for better understanding of the functions that growth factors play in the various phases of wound healing. In vitro and in vivo studies have confirmed their ability to enhance the capacity of tissues to regenerate by regulating cell chemo-attraction, differentiation, and proliferation. Studies have also examined their use for alveolar bone regeneration in periodontal, reconstructive, and pre-prosthetic surgery, including rehabilitation with dental implants.
Platelets isolated from the peripheral blood are an autologous source of growth factors. In 1974, Ross et al published one of the first papers describing the regenerative potential of platelets.3 In the general medical setting, platelets are used to prevent and treat a variety of bleeding conditions, including severe thrombocytopenia, severe oral hemorrhage, and acute leukemia. The use of fibrin glue or adhesives, which consist primarily of fibrinogen and thrombin, helped to initiate the development of platelet concentrate as a bioactive surgical additive.3 In addition, a number of blood components have been increasingly recognized as being part of the natural healing process, with the potential to accelerate wound healing when added to injured tissues or surgical sites.3
Reparation and regeneration of the soft and hard tissues after periodontal surgical procedures can be improved with the use of platelet concentrates. Growth factors are released after activation from platelets trapped within fibrin matrix, which in turn stimulates the mitogenic response in the periosteum for bone repair during normal wound healing. The growth factors listed below can be found in the environment of a blood clot:1,4
• Transforming growth factor beta (TGF-b)
• TPlatelet-derived growth factor (PDGF)
• Insulin-like growth factor (IGF)
• Vascular endothelial growth factors (VEGF)
• Epidermal growth factor (EGF)
• Fibroblast growth factor-2 (FGF-2)
Platelet-derived growth factor (PDGF), which is synthesized by platelets, monocytes, macrophages, endothelial cells, and osteoblasts, has been extensively investigated for clinical applications and appears to have broad wound-healing activities in both hard and soft tissue.4 A naturally occurring protein, it is abundant in bone matrix and is released locally by blood platelets during clotting following a soft- or hard-tissue injury. After its release from the platelets, PDGF binds to specific cell surface receptors and promotes rapid cell migration and proliferation in the injured area. Both in vitro and in vivo studies suggest that PDGF is a potent chemotactic and mitogenic factor for gingival and periodontal ligament fibroblasts, cementoblasts, and osteoblasts.
The initial idea was to concentrate platelets and their growth factors and to deliver the preparation to a surgical site in order to improve and expedite healing. Studies have demonstrated promising effects in that the use of these autologous products results in better and more rapid healing, and regeneration of both soft and hard tissue.5 Because healing is more rapid, the risk of complications such as infection, dry socket, failed implants, or failed grafting is lower. These procedures may be particularly useful for individuals who are at risk for impaired healing such as diabetics, smokers, and/or those with chronic or acute conditions that can interfere with healing.
Various methods of using autologous platelet concentrates have been developed and are being used in oral and maxillofacial surgery. The “first generation” incorporates the platelet-rich plasma (PRP), and is the precursor of another autologous derivate, the platelet-rich fibrin (PRF), which is a solid fibrin-based biomaterial.
Platelet-Rich Plasma
In 1998, Marx et al wrote about PRP,6 which essentially is an increased concentration of autologous platelets suspended in a small amount of plasma after centrifugation. These early data suggested that adding PRP accelerated the rate and degree of bone formation.
PRP was developed to combine the fibrin’s sealant properties with the growth factor effects of platelets. Its ability to accelerate wound healing is based on the combination of growth factors contained in platelets, which stimulate different stages of the wound cascade.7 PRP is basically a simple method of concentrating platelets or enriching natural blood clots, as a natural clot consists of 94% red blood corpuscles (RBCs), 5% platelets, and 1% white blood corpuscles (WBCs). In contrast, PRP is almost entirely composed of platelets (95%).6
Thus, PRP offers the advantage of supplying synergistically working growth factors to the wound site and in concentrations that are biologically and physiologically beneficial.
Currently, the use of PRP has proven to be advantageous in a number of clinical applications,5 as specified below:
• Any type of bone grafting will heal significantly faster.)
• PRP can reduce inflammation, promote more rapid bone growth, and help prevent nerve damage and dry sockets when placed in wisdom teeth sockets.)
• PRP use can speed up ridge augmentation procedures.)
• PRP can be used as fibrin glue in jaw reconstruction surgeries.)
• PRP has been shown to help fill intrabony defects or osseous defects.)
• PRP is useful in soft-tissue procedures, including gingival and subepithelial grafts.)
Platelet-Rich Fibrin
A second-generation platelet derivative, PRF was developed in France by Choukroun et al in the setting of oral maxillofacial surgery.8,9 PRF is different from other types of platelet concentrates in that it requires neither anticoagulants nor bovine thrombin or any other gelifying agent.7
PRF consists of a fibrin matrix polymerized in a tetra molecular structure, in which platelets, leucocytes, cytokines, and circulating stem cells are trapped and then slowly released. The natural fibrin architecture seems to be the component responsible for this slow release of growth factors and matrix glycoproteins over the course of 7 to 21 days; and the slow polymerization during its preparation appears to create a fibrin network that is very similar to the natural one. PRF may be considered superior to PRP, due to a simpler processing technique that does not require biochemical blood handling. In addition, some data suggest that PRF yields better results than PRP, as well as superior healing properties.8 Clinical applications of PRF include the following:
• PRF and PRF membrane used in combination with bone grafts can speed healing in lateral sinus floor elevation procedures.
• It can protect and stabilize graft materials during ridge augmentation procedures.
• After tooth extraction or avulsion, it can help to preserve the socket.
• It can be used for root coverage with single- and multiple-tooth recession.
• It can be used in regenerative procedures for a 3-walled osseous defect.
• It enhances palatal wound healing after free-gingival grafts.
Case Study
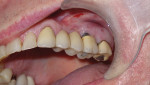
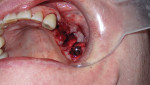
A 63-year-old male nonsmoker presented at the dental office complaining of pain in the upper left side of his mouth. His general health was good without any chronic comorbid conditions. He had a generally uneventful dental history, but had a 10-year-old bridge from teeth Nos. 11 through 15. The pain and swelling appeared to be related to a failed root canal on tooth No. 14 (Figure 1).
After taking the dental history, a full set of updated dental radiographs was taken and a complete dental examination was conducted.
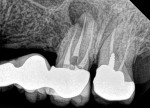
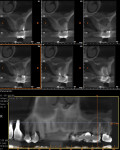
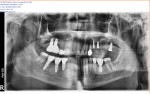
A regular periapical film revealed a large periapical pathology. Subsequent 3D rendering showed that the lesion was significantly larger than was initially apparent, affecting all of the walls of tooth No. 14 and extending into the mesial of tooth No. 15. The clinical examination revealed swelling around the tooth; a small fistula (Figure 2 and Figure 3) was also noted on tooth No. 14. The patient stated that he had already been to an oral surgeon, who recommended an apicoectomy on tooth No. 14 but did not take a computed tomography (CT) scan of the area. The author’s CT scan of teeth Nos. 14 and 15 revealed how significant the lesion actually was, and that both the buccal and palatal walls of both of those teeth were involved.
The treatment plan was to section the existing bridge—leaving tooth No. 11 intact—and to place immediate implants on teeth Nos. 12, 13, and 15, if possible, using PRF/PRP with freeze-dried demineralized bone to graft the area and repair the lesion.
In the author’s practice, PRP (liquid) and PRF therapies are considered the standard of care for implants, as patients have been found to experience faster and stronger bone regeneration with fewer complications, fewer infections, and very favorable soft-tissue healing. The procedure was explained to the patient, who readily agreed to it.
The procedure was performed under a local anesthetic. Two carpules of articaine 1/200,000 epinephrine and two carpules of lidocaine 1/200,000 were administered, and teeth Nos. 14 and 15 were surgically removed atraumatically.
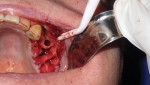
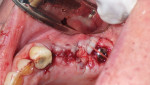
A full-thickness mucoperiosteal flap with a small vertical releasing incision was placed distally to tooth No. 15. The entire area was then completely degranulated in order to regenerate bone. Implant placement was based on an infection-free, completely degranulated site with excellent initial stability (Figure 4).
Next, 4.0-mm x 11.5-mm implants (AnyRidge®, Integrated Dental Systems, www.idsimplants.com) were placed in site Nos. 12 and 13 with initial stability above 40 Ncm; a 4.5-mm x 10-mm AnyRidge implant was placed in site No. 15 with stability above 40 Ncm. Cover screws were placed on the implants in site Nos. 12 and 13, while a 7-mm x 3-mm high healing cap was placed on the implant in site No. 15. These large healing caps facilitate primary closure, which is indicated for healing in bone-grafting procedures (Figure 4).
Four applications of PRF/PRP were made, and they were placed all together in order to facilitate the release of all growth factors, which would occur in 1 to 10 days. PRF is a clot that can be molded into a membrane for guided bone regeneration, and the growth factor release occurs mainly within 7 to 28 days (Figure 5).
PRP is injected around the wound site and mixed with allograft (bone graft) for immediate growth factor release, while PRF can be molded into a membrane for simple ridge augmentation procedures. It is cut into pieces and added to the graft, and a hole can be punched through it, allowing placement at the suture line to create primary closure for optimal healing.
In this procedure, the PRF was cut up and placed with 0.5 cc of demineralized bone and mixed with PRP (which acts as fibrin connection). It was placed as a membrane over the buccal dehiscence on tooth No. 13.
PRF/PRP was placed between the bony envelope and palatal dehiscence in site No. 14, and PRF was manipulated between the gingiva on the palate of site No. 14.
A hole was punched and stabilized with the healing cap of site No. 15. PRF/PRP was placed over the flap in the area of site Nos. 14 and 15, allowing for healing, technically, by secondary intention. The incision was sutured with no tension on flap borders, with two internal mattress sutures. Healing would occur by secondary intention in the area of site Nos. 14 and 15 because of the PRF that had been placed inside the flap. The use of PRF at the suture lines of the flap enabled primary closure to be obtained without overly releasing the flap because, unlike synthetic membranes, PRF can be exposed to the mouth (Figure 6).
The surgery took approximately 2 hours, primarily because debridement took approximately 45 minutes. The patient, who tolerated the procedure extremely well, had been given four tablets of dexamethasone the morning of surgery and was placed on 875 mg amoxicillin twice per day for 7 days, 1.5 mg plus two tablets of dexamethasone the following day, and one tablet on day 3.
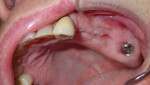
At the 1-month follow-up, the site was almost completely healed. At 4 months postoperative, the healing was complete. The patient was very satisfied and impressed with the procedure. At 1-year postoperative, no bone loss or recession was noted and there were no signs of peri-implantitis. The patient was very satisfied with the esthetics and function of his bridge. The implant was firmly in place and intact without any signs of inflammation or infection, and the patient no longer complained of pain (Figure 8 and Figure 9).
It has now been 2 years since the procedure and there are still no problems or related complications. This procedure reduced the patient’s morbidity, increased his healing time by 2 months, and avoided a future vestibuloplasty (Figure 10).
Final Thought
This case study presents a successful esthetic and functional outcome for an implant patient who received PRP and PRF therapies, which are used routinely by the author for implant cases. As was demonstrated in this instance, the author has found that patients treated in this way experience faster and stronger bone regeneration with fewer complications, fewer infections, and very favorable soft-tissue healing.
Disclosure
The author has no relevant financial relationships to disclose.
References
1. Rutkowski JL, Thomas JM, Bering L. An analysis of a rapid, simple, and inexpensive technique used to obtain platelet-rich plasma for use in clinical practice. J Oral Implantol. 2008;34(1):25-33.
2. Gabriel A, de la Torre JI, Rosenberg LZ, et al. Wound healing and growth factors. Medscape website. http://emedicine.medscape.com/article/1298196-overview. Accessed March 10th, 2016.
3. Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA. 1974;71(4):1207-1210.
4. Kim SG, Solomon C, Zheng Y, et al. Effects of growth factors on dental stem/progenitor cells. Dent Clin North Am. 2012;56(3):563-575.
5. Kaigler D, Avila G, Wisner-Lynch L, et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11(3):375-385.
6. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
7. Khiste SV, Tari RN. Platelet-rich fibrin as a biofuel for tissue regeneration. ISRN Biomaterials. 2013;2013 (627367). doi:10.5402/2013/627367.
8. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56-e60.
9. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
10. Saluja H, Dehane V, Mahindra U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofac Surg. 2011;1(1):53-57.