Restorative Advances in the Digital Era of Implantology
Diagnostic and treatment planning process based on the complexity of the case
Bradley DeGroot, DDS, MS | George A. Mandelaris, DDS, MS
Advances in implant dentistry have continued to raise the proverbial bar by which success is measured. In in the early days of dental implants, it was enough that implants were immobile, painless, had minimal (<0.2 mm) annual bone loss, and a 10-year survival rate of 80%.1 Few practitioners today, however, would be satisfied with these tenets of success. With the advent of the roughened-surface implant, survival rates have become consistently greater than 95% over a 5- to 10-year period.2 New innovations in implant body and placement protocols have driven the profession forward. Platform switching may minimize crestal bone loss, immediate implant placement reduces treatment time, and guided surgery improves placement accuracy—all without compromising implant survival.3-7 Thus, mere survival of an implant is no longer sufficient to differentiate between success and failure. An esthetic and functional final restoration is paramount and should be the driving force in most implant therapy.
The incorporation of 3-dimensional (3D) imaging into implant dentistry has been a major contributing factor in the rapidity of its advancement. Cone-beam computed tomography (CBCT) imaging enables the identification and evaluation of key anatomic structures such as the inferior alveolar nerve and the maxillary sinus.8-10 It allows clinicians to analyze alveolar ridges before implant placement or augmentation and evaluate hopeless teeth to determine if they may be candidates for immediate implant placement.11,12 CBCT imaging increases the amount of available information exponentially. However, all of the information that can be obtained by imaging is relatively meaningless without “restorative leadership.”
Personalized Treatment
Implant therapy is a prosthetically driven treatment modality. Contemporary implant therapy requires a combination of 3D imaging within a team context guided by the restorative goals. The role of surgical therapy is to support these goals. By embracing the concept of “collaborative accountability,” those providing implants are better able to ensure consistent results.13 Providers often need to do more than replace teeth. The shape and contour of the teeth, their emergence from the gingiva, and the volume and appearance of the soft tissue need to be considered in each case. These evaluations should be incorporated into the CBCT imaging to maximize the 3D information and allow for meaningful treatment planning of the case. A personalized diagnostic and treatment pathway should be developed for the patient in order to optimize interdisciplinary communication between the restorative dentist/prosthodontist, surgeon, and laboratory technician.
The scope of the diagnostic and treatment planning process is different for each patient and depends on the complexity of his or her case. Mecall proposed five “case types,” each requiring varying levels of diagnostic workup and treatment planning (Table 1).14 Once a patient’s case has been classified, the corresponding workup follows a formulaic pattern: create an ideal diagnostic wax-up and transfer the information to the CBCT planning.
Case Type 1
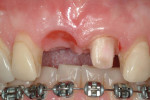
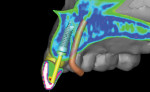
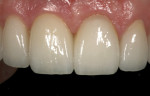
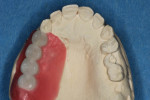
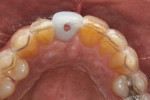
Type 1 cases are the least complicated because both the dental and surgical anatomies are within normal limits (ie, appropriate space is available for tooth replacement, adequate bone is available for ideal implant placement, and gingival esthetics are ideal). Replacement of a tooth (or teeth) can be completed without any modification of the surrounding architecture (Figure 1 through Figure 3). Thus, a diagnostic wax-up for these cases comprises the missing tooth or teeth alone. The information from this wax-up can then be transferred to the patient’s 3D planning through either the fabrication of a scanning appliance or optical imaging of the diagnostic wax-up and merging it into the 3D plan.
Case Type 2
In these types of cases the dental anatomy is within normal limits, but minor adjustments to the surgical anatomy are required. If there is early loss of the facial bone, the ideal wax-up needs to be fully contoured (replace both teeth and soft tissue). Conversely, if the phenotype is thin/discolored but spacially correct, a tooth-form wax-up may suffice. This information is then used to complete the patient’s 3D planning.
Case Type 3
Cases that are type 3 present with surgical anatomy that requires alteration while the dental anatomy remains mostly within normal limits. Often these cases require horizontal ridge augmentation and/or soft-tissue augmentation to correct the anatomic limitations and, therefore, require a fully contoured wax-up. After transferring this wax-up to the 3D image, the providers are better able to identify the most appropriate surgical and prosthetic treatment modalities.
Case Type 4
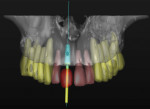
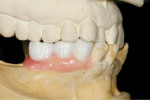
Type 4 cases are relatively complex because they involve the modification of both the dental and surgical anatomies. Such cases may present with a combination of vertical and horizontal bone loss with supraeruption, altered occlusal vertical dimension (OVD), or inappropriate space for ideal tooth form. These cases often require some vertical augmentation of the residual ridge in addition to the horizontal augmentation. As such, they require a fully contoured diagnostic wax-up at a minimum but may benefit from a trial tooth set-up if the discrepancy between actual and ideal anatomy exists in both arches (Figure 4 and Figure 5).
Case Type 5
Patients with significant dental and anatomical shortcomings fall into the final category, case type 5 (eg, an atrophic, completely edentulous ridge). These patients lack adequate tooth support to determine OVD, necessitating the fabrication of a trial tooth set-up. If the patient has an existing and well-fitting denture, the current prosthesis may either be duplicated into a differential barium gradient (30:10) scanning appliance or, more commonly, used as part of a dual-scan imaging technique.
Transferring Data From the Lab to the Scan
Traditionally, in order to transfer an ideal wax-up into 3D data, a scanning appliance would be fabricated, which is worn by the patient while the CBCT image is captured. This appliance typically is fabricated out of a radiopaque material so it can be visualized radiographically to enable case planning (Figure 6 and Figure 7). These scanning appliances can be fabricated using barium/acrylic teeth with a negative image center (Figure 6) or with acrylic teeth utilizing a positive image center (gutta percha or another radiopaque material).
More recently, 3D technology has evolved to allow the transfer of data from the wax-up without the use of a scanning appliance. With this technology, a CBCT image of the patient is first made without a scanning appliance in place. Then, a wax-up is duplicated into a stone cast, the cast is optically scanned, and this data can be merged with the original CBCT scan of the patient using planning software such as SIMPLANT® (DENTSPLY Implants, www.dentsplyimplants.com) (Figure 8 and Figure 9). Maven Pro by nSequence (www.nsequence.com) is another comparable treatment planning software that can be used in these types of cases. Patients who already have a well-fitting prosthesis can be scanned with the prosthesis in place in accordance with the dual-scan protocol. The prosthesis is then scanned alone and the two images are merged utilizing fiduciary markers attached to the prosthesis that allow for proper 3D alignment.
Conclusion
As implant dentistry has progressed, expectations have changed—of both the clinicians placing and/or restoring the implants and the patients receiving them. Implant survival is no longer the sole standard by which success is gauged; it is expected. Ideal replacement of the patient’s soft- and hard-tissue deficiencies should be the goal of anyone involved in implant dentistry. While 3D imaging has drastically increased the capability to evaluate head and neck anatomy, plan cases, and identify favorable sites for implant placement, its utility still requires “restorative leadership” for it to meet its full potential. Integration of digital technology, including implant planning software, is an emerging standard of care but is not a substitute for sound prosthetic fundamentals or biologic principles of wound healing.
Incorporating technological advances into clinical practice requires cooperative and collaborative input from all those responsible for patient care. However, it is the restorative dentist who must define and communicate the expected outcomes to the interdisciplinary team. While it is impossible to develop a one-size-fits-all approach to achieving ideal implant esthetics and function, organizing patients into case type patterns helps guide clinicians through the diagnostic phase of implant care and maximizes the potential benefits of CBCT imaging. The leadership of the restorative dentist, through an ideal wax-up or set-up, creates an environment of “collaborative accountability” by establishing and illustrating the performance standards for each case.
References
1. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
2. Buser D, Mericske-Stern R, Bernard JP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8(3):161-172.
3. Annibali S, Bignozzi I, Cristalli MP, et al. Peri-implant marginal bone level: a systematic review and meta-analysis of studies comparing platform switching versus conventionally restored implants. J Clin Periodontol. 2012;39(1):1097-1113.
4. Atieh MA, Ibrahim HM, Atieh AH. Platform switching for marginal bone preservation around dental implants: a systematic review and meta-analysis. J Periodontol. 2010;81(10):1350-1366.
5. Lang NP, Pun L, Lau KY, et al. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin Oral Implants Res. 2012;23 suppl 5:39-66.
6. Testori T, Robiony M, Parenti A, et al. Evaluation of accuracy and precision of a new guided surgery system: a multicenter clinical study. Int J Periodontics Restorative Dent. 2014;34 suppl 3:S59-S69.
7. Tahmaseb A, Wismeijer D, Coucke W, Derksen W. Computer technology applications in surgical implant dentistry: a systematic review. Int J Oral Maxillofac Implants. 2014;29 suppl:25-42.
8. Rosa MB, Sotto-Maior BS, Machado Vde C, Francischone CE. Retrospective study of the anterior loop of the inferior alveolar nerve and the incisive canal using cone beam computed tomography. Int J Oral Maxillofac Implants. 2013;28(2):388-392.
9. van Zyl AW, van Heerden WFP. A retrospective analysis of maxillary sinus septa on reformatted computerised tomography scans. Clin Oral Implants Res. 2009;20(12):1398-1401.
10. Chan HL, Monje A, Suarez F, et al. Palatonasal recess on medial wall of the maxillary sinus and clinical implications for sinus augmentation vial lateral window approach. J Periodontol. 2013;84(8):1087-1093.
11. Caldwell GR, Mills MP, Finlayson R, Mealey BL. Lateral alveolar ridge augmentation using tenting screws, acellular dermal matrix, and freeze-dried bone allograft alone or with particulate autogenous bone. Int J Periodontics Restorative Dent. 2015;35( 1):75-83.
12. Kan JY, Roe P, Rungcharassaeng K, et al. Classification of sagittal root position in relation to the anterior maxillary osseous housing for immediate implant placement: a cone beam computed tomography study. Int J Oral Maillofac Implants. 2011;26 (4):873-876.
13. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 1: diagnostics, imaging, and collaborative accountability. Int J Periodontics Restorative Dent. 2006;26(3):215-221.
14. Mecall RA. Computer-guided implant treatment pathway. In: Tardieu PB, Rosenfeld AL, eds. The Art of Computer-Guided Implantology. Chicago, IL: Quintessence Publishing; 2009:89-111.
About the Authors
Bradley DeGroot, DDS, MS
Private Practice in Periodontics
Chicago, Illinois
Park Ridge, Illinois
Oakbrook Terrace, Illinois
George A. Mandelaris, DDS, MS
Adjunct Clinical Assistant Professor
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
Private Practice in Periodontics
Chicago, Illinois
Park Ridge, Illinois
Oakbrook Terrace, Illinois