Implant Placement and Immediate Provisionalization of Edentulous Arches
A solid understanding of the biological and mechanical principles underlying the immediate-loading concept is necessary for achieving clinical success.
A growing segment of patients desire fixed implant-supported restorations to improve their smiles and dental function, as well as to enhance their appearance. Extraction with immediate implant placement and provisional restoration has become an attractive option1-4 for meeting some of the esthetic and biomechanical challenges associated with using implants to replace hopeless or missing dentition.
A solid understanding of the biological and mechanical principles underlying the immediate-loading concept is necessary for achieving clinical success. Immediately loaded dental implants must achieve adequate primary stability5-8 and should be rigidly splinted around the curvature of the arch. The provisional prosthesis should be undisturbed for a minimum of 2 months during the post-placement healing period. To increase the success and predictability of such treatment, a set of treatment guidelines (DIEM® 2, BIOMET 3i, www.biomet3i.com) was developed in conjunction with implant components that simplify the clinical application of the immediate-loading concept.9
The following case demonstrates treatment of a patient who presented with severely debilitated dentition. Following the DIEM 2 Guidelines, implants placed in both of the patient’s edentulous arches were provisionally restored immediately after implant-placement surgery.
Case Presentation
The 56-year-old man presented complaining of pain and excessive tooth mobility. He wanted to avoid complete dentures, instead desiring treatment that would provide him with fixed implant-supported restorations.
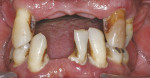
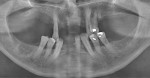
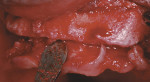
At the clinical examination, advanced generalized periodontitis was evident, along with 2+ mobility for all teeth (Figure 1). Radiographs demonstrated moderate to severe bone loss (Figure 2), with inadequate maxillary bone volume to allow for placement of dental implants immediately following extraction of the hopeless maxillary dentition. Therefore, a staged approach to treatment of the maxilla was deemed appropriate.
The patient consented to the following plan. In the first phase of treatment, the remaining maxillary teeth would be extracted, and bilateral maxillary sinus lifts and an anterior tunnel graft would be performed. A provisional maxillary full denture would be delivered immediately. This would later be converted to a fixed, implant-retained prosthesis, following the DIEM® 2 Guidelines. In the mandible, the bone volume was adequate to enable implant placement immediately after extraction, followed by delivery of a fixed provisional restoration. Although every effort would be made to maximize the interarch distance, the patient presented with relatively short alveolar processes, and interarch space was limited.
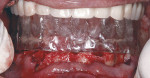
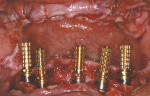
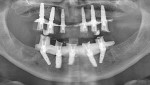
After the maxillary extractions and sinus lift/grafting, the immediate maxillary denture was placed. A mandibular surgical guide was fabricated and tried in. An alveolectomy was accomplished consistent with the known surgical volume required for implant primary stability (Figure 3). One NanoTiteTM Tapered PREVAIL® Implant and four NanoTite Tapered Implants (BIOMET 3i) were placed consistent with the treatment plan in the positions of tooth Nos. 20, 22, 25, 27, and 29. The two posterior implants were placed with a distal tilt. This increased the anterior-posterior (AP) spread. The use of 30º Angled Low Profile Abutments (BIOMET 3i) compensated for the distal tilts of the posterior implants. Because no angle correction was needed for the three anterior implants, straight Low Profile Abutments with 2-mm collar heights were selected (Figure 4). Because of the minimal restorative volume in the mandible, Low Profile Abutments were used to minimize the amount of restorative volume occupied by the implant abutments. Figure 5 shows the dimensions and configurations of Low Profile Abutments. All abutment screws were torqued to 20 Ncm with a torque-indicating device.
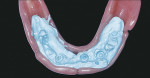
Quick-setting polyvinylsiloxane occlusal registration material was injected into the intaglio surface of the immediate denture, and the prosthesis was inserted with the patient in centric occlusion. The locations of the implants were identified in the impression (Figure 6). Holes were drilled through the denture to facilitate attachment of the temporary cylinders to the prosthesis. Low Profile Abutment Non-Hexed Temporary Cylinders were placed onto the abutments (Figure 7); their heights were adjusted extraorally so that these fit within the confines of the occlusal surfaces of the denture teeth and did not interfere with the vertical dimension of occlusion. A rubber dam was placed around the mandibular abutments and cylinders (Figure 8). This isolated the surgical and prosthetic fields. The mandibular prosthesis was adjusted to provide clearance for the temporary cylinders. The immediate mandibular denture was converted into a fixed provisional restoration by injecting autopolymerizing acrylic resin into the intaglio surface of the prosthesis and around the temporary cylinders. The patient was guided into centric occlusion, and the resin was allowed to set. The prosthesis was removed by unscrewing the retaining screws.
Polishing protectors were placed onto the abutment restorative platforms of the cylinders, and the prosthesis was finished and polished. It was inserted back onto the Low Profile Abutments with the retaining screws (torqued to 10 Ncm). The screw-access openings were restored with light-cured composite resin.
The patient left the surgical office with an immediate maxillary complete denture and a fixed mandibular implant-retained provisional prosthesis in place (Figure 9 and Figure 10). The mandibular prosthesis was supported by five implants. Each implant had an insertional torque of at least 50 Ncm and was considered primarily stable. The vertical dimension of occlusion was not changed from the patient’s original presentation. He was discharged in excellent condition and was scheduled to return in 10 days for the first postoperative visit.
Two months later, the patient returned for the second phase of treatment. This included placement of six maxillary implants, immediately followed by conversion of the existing complete maxillary denture to a fixed, implant-retained prosthesis, following the DIEM® 2 Guidelines.
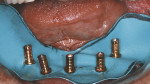
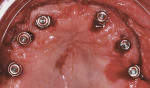
The edentulous maxilla had healed with a broad, U-shaped ridge and had adequate fixed keratinized tissues. A surgical guide that was fabricated by duplicating the maxillary complete denture in clear acrylic resin was seated with a laboratory-fabricated interocclusal record (Figure 11). A full-thickness flap with a vestibular incision was reflected. Four NanoTite™ Certain® Tapered Implants and two NanoTite Tapered PREVAIL® Implants were placed, each with insertional torque values of at least 50 Ncm. Straight-collar One-Piece Low Profile Abutments (2-mm and 3-mm heights) were placed into the internal interface of the implants and secured with abutment screws, which were tightened to 20 Ncm of torque using the Standard Abutment Driver Tip (RASA3) and a torque device (Figure 12). A quick-setting polyvinylsiloxane occlusal registration material was injected into the intaglio surface of the maxillary denture. The denture was placed into the mouth with the occlusal record to guide the patient into centric occlusion. The material was allowed to set and the denture was removed. The locations of the implants relative to the denture teeth were identified. Holes were drilled through the prosthesis, and the prosthesis was set aside.
Low Profile Abutment Non-Hexed Temporary Cylinders were placed onto the abutments with retaining screws. These were hand-tightened, and the cylinders were adjusted extraorally and placed back onto the abutments (Figure 13). The prosthesis was adjusted so that there was no contact with the temporary cylinders. A rubber dam was placed around the temporary cylinders that separated the surgical and prosthetic fields. The same autopolymerizing resin was used as described for the fabrication of the mandibular prosthesis. The pre-existing vertical dimension of occlusion was maintained during this portion of the procedure. The prosthesis was removed by releasing the retaining screws. Polishing protectors were placed onto the abutment restorative platforms, and the prosthesis was finished and polished.
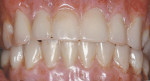
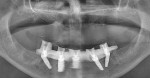
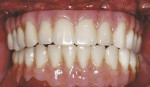
The patient left the surgical office with a fixed maxillary implant-retained prosthesis (Figure 14 and Figure 15). At the time of initial implant placement, each implant had an insertion torque of at least 50 Ncm and was considered primarily stable. The vertical dimension of occlusion was not changed from the patient’s original presentation. He was discharged in excellent condition.
Conclusion
The complex treatment described here is a perfect example of the cooperation necessary among the dental implant team, which in this case consisted of a surgeon, restorative dentist, and dental laboratory technician. The implants were first placed in the mandible. Traditionally mandibular bone is denser than maxillary bone; implants placed typically have insertion torques in excess of 50 Ncm. This patient’s preoperative maxilla did not have adequate bone for immediate implant placement postextraction. Bone grafting (bilateral sinus lifts) was needed in the posterior segments. After osseous healing, the maxillary bone was dense enough to provide implant primary stability at the time of implant placement. The maxillary implants achieved insertion torques similar to those achieved for the mandibular implants. The maxillary complete denture was converted into a fixed, implant-retained prosthesis using the same protocol as the mandibular prosthesis. Although the placement of four rigidly splinted implants has been shown in the literature to be highly successful, the requirements for each patient must be carefully evaluated. Several factors need to be considered when treatment planning these complex cases, including bone quality and quantity, AP spread, occlusal function of the patient, and skeletal patterns.
Disclosure
The authors are paid consultants and speakers for Biomet 3i.
References
1. Schnitman PA, Wöhrle PS, Rubenstein JE, et al. Ten-year results for Brånemark implants immediately loaded with fixed prostheses at implant placement. Int J Oral Maxillofac Implants. 1997;12(4):495-503.
2. Colomina LA. Immediate loading of implant-fixed mandibular prostheses: A prospective 18-month follow-up clinical study—preliminary report. Implant Dent. 2001;10
(1):23-29.
3. Tarnow DP, Emtiaz S, Classi A. Immediate loading of threaded implants at stage 1 surgery in edentulous arches: Ten consecutive case reports with 1- to 5-year data. Int J Oral Maxillofac Implants. 1997;12(3):319-324.
4. Testori T, Szmuckler-Moncler S, Francetti L, et al. Immediate loading of Osseotite implants: A case report and histologic analysis after 4 months of occlusal loading. Int J Periodont Rest Dent. 2001;21:451-459.
5. Szmuckler-Moncler S, Piatelli A, Favero GA, Dubruille JH. Considerations preliminary to the application of early and immediate loading protocols in dental implantology. Clin Oral Impl Res. 2000;11(1):12-25.
6. Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Act Orthop Scan Suppl. 1993;255:1-58.
7. Vaillancourt H, Pilliar RM, McCammond D. Finite element analysis of crestal bone loss around porous-coated dental implants. J Appl Biomater. 1995;6(4):267-282.
8. Brunski JB. Biomechanical factors affecting the bone-dental implant surface. Clin Mater. 1992;10(3):153-201.
9. Lazzara RJ, Testori T, Meltzer A, et al. Immediate occlusal load (IOL) of dental implants: predictable results through DIEM guidelines. Pract Proced Aesthet Dent. 2004;
16(4):3-15.
About the Authors
Brent Boyse, DDS
Private Practice
Mesa, Arizona
Sheldon Sullivan, DDS
Private Practice
Mesa, Arizona