You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The esthetic enhancement of the natural dentition is a significant component of the contemporary dental practice. Proper zones of attached/keratinized tissue can lead to a balanced, harmonious gingival complex that can complement the ceramic alteration of the natural tooth, or tooth replacement with dental implants. In areas where there is a lack of attached keratinized tissue, in addition to root surface exposure, consideration must be given to correcting the deficient tissue contours before any tooth alteration and/or implant placement procedures.1,2 Various procedures to correct deficient gingival contours have been well documented in the dental literature.3,4
Increasing zones of attached gingiva using palatal donor tissue and the free gingival grafting procedure was introduced by Bjorn almost a half century ago.5 Using palatal donor tissue in the form of a free soft tissue autograft for root coverage procedures was reported by Miller.6 Additional procedures were reported using lateral7 or coronal8-10 repositioning of the adjacent attached gingivae via a pedicle flap, or the coronal repositioning of previous grafted tissue.11-12 Miller also reported gingival grafts placed over root surfaces to correct areas of deep-wide gingival recession.13 Further surgical advancements led to using subepithelial connective tissue from the palate to obtain root coverage.14-16
One of the impediments to patients accepting soft tissue procedures to correct gingival loss is the trauma from harvesting palatal donor tissue. Depending on the volume of tissue required to correct the recession present, multiple harvesting procedures may be required. Also, an inadequate amount of connective tissue may be present, and the patient’s medical status may play a role in being a good candidate for palatal donor site surgery. As a result of some of these concerns, corrective gingival surgery expanded to the use of acellular dermal matrix grafts as a substitute for palatal connective tissue grafts.17-19 Harris reported a comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft.20 He observed no clinical or statistical difference between the two materials. Henderson et al reported on predictable multiple-site root coverage using an acellular dermal matrix autograft,21 with additional clinical documentation of dermal matrix grafts and their successful use in root coverage procedures.22-25 Allen described a tunneling technique during which a surgical pouch is created, the acellular dermal matrix is placed into the pouch, and the pouch is then coronally repositioned to cover the graft completely.26
Obvious advantages exist in using acellular dermal matrix grafts. The avoidance of harvesting the palatal tissues is a major benefit to patients undergoing this type of treatment. For the surgeon, to have unlimited amounts of tissue available, and to be able to treat multiple teeth/sites at one surgical visit, makes the surgical procedure more efficient and less traumatic.26 Additionally, a high quality of the donor tissue, in addition to its natural esthetic appearance, and patients’ improved acceptance of therapy makes this tissue an ideal replacement procedure for palatal soft tissue grafting.
There are two sources of acellular dermal matrix grafts available to dental professionals; both are represented in this article. As documented in the dental literature, AlloDerm (Biohorizons, Inc, www.biohorizons.com) has been in use for more than 15 years with success, whereas the alternate product, Puros® Dermis Allograft Tissue Matrix (Zimmer Dental, www.zimmerdental.com), has only case reports documenting its use to date. Both will be reviewed here.
AlloDerm RegenerativeTissue Matrix
AlloDerm is an acellular dermal matrix derived from donated human skin that undergoes a multistep proprietary process that removes both the epidermis and the cells that can lead to tissue rejection. AlloDerm’s use has been well documented in the dental literature.27-32 AlloDerm is available in two thicknesses for use in various procedures: 0.9 mm to 1.6 mm for root coverage and soft tissue ridge augmentation; and 0.5 mm to 0.8 mm for guided bone regeneration and barrier membrane function. AlloDerm has been used for more than 15 years with an excellent safety history, and has been used in more than 1 million procedures ranging from general, urogenital, orthopedic, and dental surgeries.33
In the procurement and safety process33 of AlloDerm, tissue is accepted from tissue banks in compliance with the American Association of Tissue Banks guidelines, and processing technology removes immunologic cells and decreases the risk of disease transmission. The tissue then goes through a panel of serologic tests; final testing ensures that no pathogens are introduced throughout the processing method.
Processing of AlloDerm
The processing of AlloDerm from donor tissue is a proprietary process that involves a series of steps that removes the epidermis and cells in the dermis that can lead to graft rejection and/or failure or recipient responses. The extracellular matrix that remains is then put through a proprietary freeze-drying step that preserves the tissue without damage from ice crystal formation. Histologic testing is then done on each final lot to verify cell removal.34
Puros Dermis Allograft Tissue Matrix
Puros® Dermis is recovered following the rigorous standards of both the Food and Drug Administration and the American Association of Tissue Banks (AATB) with either a scalpel or dermatome from the back of the thighs of the donor. The tissue is recovered within 24 hours of death using an aseptic process by a recovery team that meets the standards set by the AATB. The tissue does not enter the Tutoplast® Process (Tutogen Medical/RTI Biologics, Inc; www.rtix.com) until it passes serological tests, such as human immunodeficiency virus, hepatitis, human T-lymphotropic virus, and syphilis.35 The Tutoplast® Process is a multistep process36 that removes all antigenicity, inactivates all kinds of pathogens, preserves tissue structure and collagen, preserves biomechanics, guarantees sterility, and results in graft healing comparable to autografts. The process itself consists of donor selection, osmotic treatment, oxidative treatment, alkaline treatment (different from bone), solvent dehydration, low-dose gamma irradiation, and full documentation.
The Tutoplast® Process is fully validated for biomechanics, virus inactivation, prior inactivation, antigenicity, and healing. The Puros Dermis Tissue allograft has a strength-to-failure measurement of 5 pounds ± 0.8, can be stored at room temperature, has a 5-year shelf life, and, due to the Tutoplast process, has no residual chemicals as it is packed and delivered to the clinician. Compared to the dermal matrix available on the market that has a failure measurement of 4.5 pounds ± 1.1, must be refrigerated, has only a 2-year shelf life, and is packed with a variety of residual antibiotics, the Puros Dermis Tissue, in the author’s opinion, offers a superior graft product to the clinician.
Enhancement of the Dermal Matrix with Platelet Rich Plasma
The enhancement of bone and tissue grafts with platelet-rich plasma (PRP) has been well documented in the dental literature.35-39 PRP (autologous platelet gel) is developed from autologous blood with a cell separator. A 60-mL syringe is prefilled with 5 mL of a citrate-based anticoagulant (ACD-A). For each 60-mL syringe, approximately 45 mL to 55 mL of the patient’s blood is withdrawn from a venous puncture in the upper arm. The anticoagulated blood is dispensed into the blood chamber of the processing disposable. The blood must be drawn before the commencement of surgery, because surgery itself leads to platelet activation of the coagulation system.
A processing disposable is loaded into the centrifuge rotor cup of the Smart PReP® Platelet Concentrate System (Terumo; www.terumo-us.com). A counterbalance is then placed in the opposing rotor cup unless a second processing disposable is required. During processing, the blood is initially centrifuged at 3,650 rpm to separate the red blood cells from the plasma. The centrifuge then slows to approximately 60 rpm, allowing the plasma to automatically decant into the plasma chamber. The centrifuge then accelerates to 3,000 rpm to form a pellet of pure plasma concentrate in the bottom of the plasma chamber. The entire process of separating the whole blood into red blood cells, platelet-poor plasma (PPP), and platelet concentrate is completely automatic and completed in approximately 12 minutes.
The blood chamber of the process disposable contains red blood cells. The second chamber contains the platelet concentrate (a button-like precipitate) and PPP (supernatant). Approximately two thirds of the PPP are removed and can be saved for hemostatic applications. The platelet concentrate is then resuspended in the remaining PPP, thereby creating a very concentrated PRP solution.
The activator for the PRP and PPP is a mixture of 5,000 units of topical bovine thrombin and 5 mL of 10% calcium chloride. The activator is drawn into two 1-mL syringes and 10 mL of PRP is then drawn into one syringe and 10 mL of PPP is drawn into the other syringe. The two syringes are attached to a 20-gauge dual cannula applicator tip where the contents are mixed as they are applied into/onto the bone graft, wound, connective tissue graft, or incisions.
The following growth factors are found in PRP: platelet-derived growth factor; transforming growth factor-beta; platelet-derived endothelial cell growth factor; platelet-derived angiogenesis factor; insulin-like growth factor; and vascular endothelial growth factor.35-36 Their effects have been suggested to reduce edema and discomfort while promoting more rapid healing.37-39
As dentistry continues to expand into the esthetic realm, whether enhancement of the natural tooth, or replacement of the tooth system with dental implants, the soft tissue contours established and maintained play a major role in the success of the reconstruction and/or cosmetic procedure. Pre-prosthetic gingival surgery can enhance the final result in reconstructive and cosmetic dentistry. As the popularity of dental implants as a tooth replacement option continues, especially in the esthetic zone, the necessity of a thick biotype of soft tissue,40 in addition to attached keratinized gingiva,41 is important to increase the predictability of the procedure. Additionally, incorporating prosthetic principles at the time of surgery can enhance the esthetic aspect of the procedure by using those principles to sculpt and guide tissue contours.42-46
The following clinical cases will document the use of acellular dermal matrix grafts around natural teeth and dental implants to augment insufficient gingival contours.
Case 1
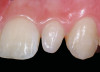
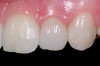
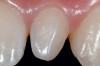
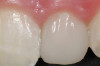
A 54-year-old non-smoking woman presented for correction of deep-wide gingival recession in the maxillary anterior (Figure 1 through Figure 3). The patient’s desires were to correct the gingival recession, balance the heights of contour of the tissues, and possibly undergo esthetic enhancement of the maxillary anterior with veneer restorations.
The treatment plan was to increase the zone of keratinized tissue in addition to root coverage in the maxillary anterior. The patient was given the option of using her palatal tissue as a donor site or the use of acellular dermal matrix tissue; she opted for the latter as the graft to be used.
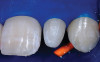
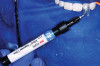
Following the technique outlined for fabrication of PRP, blood was harvested from the patient’s anticubital fossa, and the process initiated. The dermal matrix tissue was then trimmed for each of the surgical sites to be treated. The horizontal dimension of the graft should extend 2 mm to 3 mm beyond the last tooth where recession is present at each end of the surgical site, whereas the vertical dimension should be 6 mm to 8 mm. Once the graft was properly trimmed and sized, it was then submerged in a solution of non-activated PRP for rehydration. This was accomplished while the surgical site was being prepared (Figure 4).
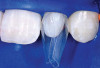
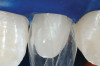
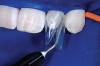
After administration of an appropriate local anesthetic, root preparation was accomplished by scaling and root planing with hand instrumentation and rotary instruments, followed by burnishing of the roots with 24% ethylene diaminetetra acetate (EDTA) and citric-acid solution (pH1) (Figure 5 and Figure 6).
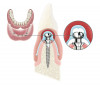
Following the surgical technique previously referenced26 with some modifications, intrasulcular incisions were made around each tooth to be treated to a minimum of one tooth on each side of the affected teeth. The incision was not carried through the entire papillae apical to the contact point, roughly being contained between the mesial and distal line angle of each affected tooth. After the incisions were made, instruments to create a tunnel under each of the incised portions of the papillae were utilized to elevate the base of the papillae. The pouch was then created by blunt dissection using a mucoperiosteal elevator extending the reflection apically past the mucogingival junction, and laterally to the facial aspect of the tunneled papillae. Occasionally, the papillae may separate in this process, as was the situation in this case. Deepening and mobilization of the pouch was then accomplished by sharp supraperiosteal dissection, which would allow for the pouch to be coronally advanced and cover the dermal tissue completely.
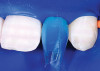
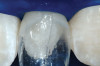
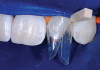
The AlloDerm material, which had been re-hydrated and enhanced with the non-activated PRP solution, was then placed into the surgical pouch with the basement membrane adjacent to the root surface (Figure 7). The dermal matrix was then secured with 6.0 polypropylene sutures (Figure 8). The graft should be positioned at the cemento-enamel junction. The pouch was then coronally advanced to cover the dermal matrix graft completely, and secured with 5.0 monocryl or 6.0 polypropylene sutures (Figure 9). The sutures were removed 1 month postoperatively; note that the need for a topical anesthetic may be necessary for patient comfort during suture removal.
The 2.5-week postoperative clinical view can be seen in Figure 10 through Figure 12. Note the rapid soft tissue healing and maturation. At 6-weeks postoperative, tissue plasty was accomplished to blend the thickened keratinized tissue, in addition to placement of class V composite restorations at teeth Nos. 5, 6, and 11 to create a new restorative margin on the root surfaces.
The 2-month postoperative view can be seen in Figure 13 through 15. Note the color match of the tissue, balance of the facial heights of contour, and zones of attached keratinized tissue present.
Case 2
A 27-year-old non-smoking man presented for esthetic enhancement of the left central incisor, which had a pre-existing full-coverage restoration that required replacement (Figure 16). The patient had begun orthodontic treatment in the mandibular arch to correct a minor occlusal imbalance and required coronal repositioning and soft tissue grafting to correct gingival recession at the facial of the right canine, in addition to correction of the free gingival margin at the facial of the left central incisor, which was asymmetrical to the free gingival margin of tooth No. 8. Additionally, the facial gingival tissues were of a thin biotype, which at the facial surface of the left central incisor allowed for the darkened root surface to be visible through the tissue (Figure 16).
The treatment plan was to accomplish an increased zone of keratinized tissue at tooth No. 6, and increase the thickness of the facial tissue at tooth No. 9, in addition to coronally repositioning the free gingival margin. Complicating this treatment plan was the fact that at the facial surface of tooth No. 9, a biologic width invasion was present due to the prepared margin for the pre-existing restoration being placed too close to the facial alveolar crest of bone (the patient had presented to the periodontist with a provisional restoration in place). Any correction to the area would require movement of the facial margin of the tooth in a coronal fashion to re-establish the appropriate distance from the facial height of bone to the margin of the restoration. Additionally, options were given to the patient for harvesting the soft tissue, using connective tissue from the palate, or the use of an acellular dermal matrix graft, which would nullify harvesting tissue from the palate region.
The patient opted for the use of the acellular dermal matrix material for the procedure, along with PRP rehydration to aid in the revascularization of the graft. The tissue selected for the augmentation procedure at teeth Nos. 6 and 9 was Puros Dermis Allograft Tissue Matrix. After an appropriate local anesthetic was administered, correction of the gingival tissue at the facial of tooth No. 6 was accomplished in a fashion consistent with the soft tissue grafting technique that will be described.
Following removal of the provisional restoration, further examination of the prepared facial margin could be made. The sounding measurements that were obtained demonstrated a distance from the free gingival margin to the crest of bone, at the mesial-facial point, to be 2 mm, the mid-facial point, 2.5 mm, and the distal-facial point, 3 mm. Normally, crown lengthening could manage this problem; however, due to the esthetic and periodontal requirements of the esthetic dentist as well as the patient, the existing margin of the tooth preparation needed to be moved in a more coronal fashion. The procedure required movement of the facial margin of the tooth preparation coronally, by root planing the existing margin off, and re-preparing a new facial margin, 1 mm more coronally, followed by dermal matrix grafting to increase the zone of tissue thickness, in addition to coronally repositioning the facial gingival margin.
Before incision, the trimmed acellular dermal matrix was rehydrated in the PRP solution previously obtained. A broad-based, papillary spacing incision was made at the facial of tooth No. 9, followed by a full-thickness, mucoperiosteal flap elevation. The facial root surface of the tooth exhibited notches and an uneven root surface. After extensive root planing of the facial root surface with hand planing and rotary instrumentation to eliminate not only the notched root surface but also the pre-existing facial tooth preparation margin, a new chamfer margin was prepared in the tooth 1 mm from the pre-existing margin (Figure 17). This was followed by application of a citric acid solution with a pH of 1 over the facial root surface for 1 minute to aid in sterilization of the root surface.6 Adjustment to the facial aspect of the pre-existing provisional restoration by shortening the facial margin by 1 mm was accomplished, and the provisional restoration was cemented with temporary cement (Figure 18).
The outline of the pre-existing facial margin can be seen in Figure 18, approximately 0.75 mm from the facial aspect of the temporary restoration. Also note the symmetry between the facial aspect of the clinical crown on tooth No. 8, and provisional restoration of tooth No. 9. Preceding fixation of the dermis, PRP was applied. The PRP-enhanced dermis was then placed over the root surface (Figure 19) and secured with 4.0 vicryl sutures in a sling fashion around tooth No. 9. Additional application of PRP preceded a tension-free flap closure, totally covering the dermis with the pre-existing facial gingival tissues. Closure was accomplished with 4.0 vicryl sutures using a horizontal mattress and continuous sling suturing method. Additional application of PRP over the surgical site served as a bioactive wound dressing.37-39
Figure 20 shows the completed view with the final restoration seated. Observe the balance of the facial heights of contour between the central incisors, and the presence of a thick-biotype, attached keratinized tissue.
Case 3
A 24-year-old non-smoking man presented for treatment of a horizontally fractured left central incisor (Figure 21 and Figure 22). The patient requested to have an implant placed to replace the left central incisor as to not have any damage to the adjacent dentition.
Complicating the treatment site was the apically positioned facial height of contour at the left central incisor, and the adjacent left lateral incisor (Figure 21.) Additional sites of recession were present throughout the oral cavity and were to be addressed by the tunnel grafting procedure previously outlined.
After reviewing treatment options that included:
- tooth removal, ridge preservation, healing phase, implant placement, healing phase, abutment connection, and removable partial denture at provisional.
- tooth removal, implant placement, bone grafting (non-immediate provisional technique), using a removable partial denture as provisional, healing phase, then stage II.
- tooth removal, implant placement, bone grafting, abutment connection and immediate provisional restoration with simultaneous soft tissue augmentation with dermal matrix tissue and the tunnel graft procedure, all by minimally invasive means.
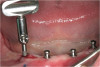
The patient opted for the third option. After administration of an appropriate local anesthetic, a frenectomy was performed using a Nd:Yag laser. This would allow for the loosening of the facial tissues and the coronal repositioning of the pouch at the termination of the procedure. After the frenectomy, the left central incisor was removed by an atraumatic technique preserving the soft tissue emergence profile (Figure 23). Debridement of the extraction socket preceded atraumatic site preparation techniques. A 3.5-mm diameter by 13-mm tapered implant (Prima Connect, Keystone Dental, www.keystonedental.com) was placed to the appropriate depth measurements planned.38,39
Once the implant was seated, the cover screw was placed, and the facial defect at the buccal aspect of the implant was corrected using mineralized, large-particle cancellous chips (Lifenet, www.lifenet.org) and a solution of PRP, forming a graft/PRP gel complex. The graft complex was heavily condensed into the void present, to the level of the facial aspect of the polished collar on the implant (Figure 24).
After placement of the graft complex, a titanium abutment with a 1-mm collar (Quick abutment, Keystone Dental) was seated and hand-tightened (Figure 25). Retrofitting of the natural tooth shell preserving the pre-existing contact point relationships and line-angle positioning was accomplished from a pretreatment incisal edge registration. The final provisional restoration can be seen in Figure 26.
Creation of the facial pouch was accomplished as outlined earlier. Intrasulcular incisions were made from the distal line angle of the right central incisor to the distal line angle of the left canine, with care given to not totally incise the papillae at the interproximal aspect. Using mucoperiosteal elevators and blunt dissection, the pouch was extended apically beyond the mucogingival junction, and laterally to the facial aspect of the tunneled papillae. The pouch was then deepened and mobilized by sharp supraperiosteal dissection. The pre-trimmed acellular dermal matrix (AlloDerm) rehydrated with non-activated PRP, was then placed into the pouch superior to the allogenic bone graft placed to correct the facial defect at the implant site, from the mesial of the right central to the mesial of the left canine (Figure 27). The acellular dermal matrix was then secured with 6.0 polypropylene sutures, and the pouch coronally advanced to cover the dermal matrix graft completely, using 5.0 monocryl sutures by an interrupted sling suturing technique (Figure 28).
The provisional restoration was an immediate non-functional restoration, and was free of contact in centric occlusion, protrusive, and right and left lateral excursive movements. Figure 29 is the 6-week postoperative view. Note the free gingival margin at the mid-facial point of the left central incisor, and compare that to the pretreatment level seen in Figure 21. The site would be allowed to heal and mature for an additional 6 weeks prior to completion of the final implant-supported restoration.
Conclusion
Adequate zones of keratinized, attached tissue are important for long-term periodontal health and maintenance. Restorative and/or cosmetic dental procedures benefit from having this type of periodontal environment. Soft tissue grafting and augmentation procedures have been developed and perfected over the last 30 years, and incorporation of acellular dermal matrix grafts have simplified the procedure, and made it more patient-friendly, allowing patients who have avoided the palatal donor harvesting procedure to have the procedure accomplished by using a safe and effective biomaterial.26 Acellular dermal matrix tissue has proven to be equal to palatal connective tissue for root coverage procedures in randomized, controlled clinical studies.12,16-19
Dermal matrix grafting possesses distinct advantages over palatal connective tissue:
- Avoidance of the palatal donor surgical site
- Multiple teeth can be treated at one visit
- Unlimited amounts of donor tissue available
- High quality of the donor tissue
- Ability to match, or be superior to, the results observed with autogenous palatal tissue grafts
Reestablishing the proper soft tissue quality before restorative intervention contributes to more predictable outcomes for tooth enhancement and replacement procedures. Acellular dermal matrix grafts provide a safe, reliable option to palatal donor connective tissue.
References
1. Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend Contin Educ Dent. 1983;4(5):437-453.
2. Abrams L. Augmentation of the deformed residual edentulous ridges for fixed prosthesis. Compend Contin Educ Dent. 1980;1(3):205-213.
3. Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part II. Prosthetic/periodontal interrelationships. Compend Contin Educ Dent. 1983;4(6):549-562.
4. Garber DA, Rosenberg ES. The edentulous ridge in fixed prosthodontics. Compend Contin Educ Dent. 1981;2(4):212-223.
5. Björn H. Free transplantation of gingival propria. Sven Tandlak Tidskr. 1963;22:684-689.
6. Miller PD Jr. Root coverage using a free soft tissue autograft following citric acid application. Part I: technique. Int J Periodontics Restorative Dent. 1982;2:65-70.
7. Bjorn H. Coverage of denuded root surfaces with a lateral sliding flap. Use of free gingival grafts. Odontol Revy. 1971;22:37-44.
8. Guinard EA, Caffesse RG. Treatment of localized gingival recessions. Part III. Comparison of results obtained with lateral sliding and coronally repositioned flaps. J Periodontol. 1978;49:457-461.
9. Tarnow DP. Semilunar coronally repositioned flap. J Clin Periodontol. 1986;13:182-185.
10. Allen EP, Miller PD Jr. Coronal positioning of existing gingiva: Short term results in the treatment of shallow marginal tissue recession. J Periodontol. 1989;60:316-319.
11. Bernimoulin JP, Luscher B, Muhlemann HR. Coronally repositioned periodontal flap. Clinical evaluation after one year. J Clin Periodontol. 1975;2:1-13.
12. Matter J. Free gingival graft and coronally repositioned flap. A 2-year follow-up report. J Clin Periodontol. 1979;6:437-442.
13. Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5(2):14-37.
14. Raetzke PB. Covering localized areas of root exposure employing the “envelope” technique. J Periodontol.1985;56:397-402.
15. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715-720.
16. Langer B, Calagna L. The subepithelial connective tissue graft. A new approach to the enhancement of anterior cosmetics. Int J Periodontics Restorative Dent. 1982;2(2):22-33.
17. Cummings LC, Kaldahl WB, Allen EP. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J Periodontol. 2005;76:178-186.
18. Silverstein LH, Callan DP. An acellular dermal matrix allograft substitute for palatal donor tissue. Post Grad Dentistry. 1997;3(4):14-21.
19. Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Periodontol. 1998;69(11):1305-1311.
20. Harris R. A comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft: Results of 107 recession defects in 50 consecutively treated patients. Int J Periodontics Restorative Dent. 2000;20:51-59.
21. Henderson RD, Greenwell H, Drisko C, et al. Predictable multiple site root coverage using an acellular dermal matrix allograft. J Periodontol. 2001;72:571-582.
22. Paolantonio M, Dolci M, Esposito P, et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: A comparative 1-year clinical study. J Periodontol. 2002;73:1299-1307.
23. Novaes AB Jr, Grisi DC, Molina GO, et al. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001;72:1477-1484.
24. Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73:1405-1411.
25. Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72:998-1005.
26. Allen EP. AlloDerm: An effective alternative to palatal donor tissue for treatment of gingival recession. Dentistry Today. 2006;25(1):48-52.
27. Santos A, Goumenos G and Pascual A. Management of gingival recession by the use of an acellular dermal graft material: A 12-case series. J Periodontol. 2005;76(11):1982-1990.
28. Paolantonio M, Dolci M, Esposito P, et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: A comparative 1-year clinical study. J Periodontol. 2002;73(11):1299-1307.
29. Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72(8):998-1005.
30. Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession. A systematic review. Ann Periodontol. 2003;8:303-320.
31. Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73:1405-1411.
32. Woodyard JG, Greenwell H, Hill M, et al. The clinical effect of acellular dermal matrix on gingival thickness and root coverage compared to coronally positioned flap alone. J Periodontol. 2004;75:44-56.
33. Data on file. LifeCell Corporation, Branchburg, NJ.
34. Data on file. Tutogen Medical, Alcoa, FL.
35. Lynch SE, Genco, Marx RE (eds): Tissue Engineering: Applications in Maxillofacial Surgery and Periodontics. Quintessence Publishing Co: 1999;71-82.
36. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
37. Petrungaro PS. Immediate restoration of dental implants after tooth removal: a new technique to provide esthetic replacement of the natural tooth system. International Magazine of Oral Implantology. 2001;2(2):6-16.
38. Petrungaro PS. Platelet-rich plasma for dental implants and soft-tissue grafting. Dental Implantology Update. 2001;11(6):41-47.
39. Garg AK. The use of platelet rich plasma to enhance the success of bone grafts around dental implants. Dent Implantol Update. 2000;11:17-21.
40. Kois JC. Predictable single-tooth peri-implant esthetics: Five diagnostic keys. Compend Contin Educ Dent. 2004;25(11):895-905.
41. Petrungaro PS. An update on implant placement and provisionalization in extraction, edentulous and sinus grafted sites: A clinical report on 3200 sites over 8 years. Compend Contin Educ Dent. 2008; 29(5): 288-300.
42. Tarnow DP, Magne AW, Fletcher P. The effect from the distance from the contact point to the crest of one on the presence or absence of interproximal dental papillae. J Periodontol. 1992;53(12):995-996.
43. Tarnow DP, Cho SC, Wallace S. The effect of inter-implant distance on the height of the inter-implant bone crest. J Periodontol.2000;
71(4):546-549.
44. Salama H, Salama MA, Garber D, et al. The interproximal height of bone: The guidepost to predictable aesthetic strategies and soft tissue contours in anterior tooth replacement. Pract Periodontics Aesthet Dent. 1998;10(9):1131-1141.
45. Petrungaro PS. Immediate restoration of implants utilizing a flapless approach to preserve interdental tissue contours. Pract Periodontics Aesthet Dent. 2005;17(2):A-H.
46. Petrungaro PS. Creation and preservation of natural soft tissue emergence profiles around dental implants in the esthetic zone. J Cosmetic Dent. 2009;24(4):66-80.