Encountering Dentin Sensitivity Before or During Esthetic Treatment
Richard Trushkowsky, DDS
Unfortunately, dentin sensitivity is a common condition that has a variety of causes and stimuli. Loss of enamel and cementum results in exposure of dentin to the oral environment and pain. As more tubules are exposed, increased dentin sensitivity occurs.1 The cause may be recession as a result of toothbrush abrasion or tooth preparation and tooth impressioning techniques. Occlusal discrepancies may possibly cause bruxing, abfraction lesions, wear (attrition), or cracked tooth syndrome. Periodontal surgery also can result in dentin sensitivity. In-office and home bleaching also can result in increased sensitivity. Erosion may be a result of extrinsic or intrinsic acids and result in sensitivity. These exogenous stimuli can include thermal changes (hot or cold), sensitivity to touch or percussion, and osmotic changes such as sweets or drying of the tooth surface.2,3 Esthetic dental procedures can be the cause of sensitivity or can be used to ameliorate or eliminate it. Ideally, the cause of the sensitivity should be recognized and the causes eliminated if possible before treatment. This article will discuss and illustrate some of the causes and treatment by presenting several cases.
Erosion has been defined as tooth dissolution by acids that are not of bacterial origin. Erosion results in the loss of tooth structure as a result of the loss of calcium and phosphate ions removed from the tooth surface. Erosion can work in conjunction with abrasion from toothbrushing with toothpaste to result in more tooth structure loss than from either effect alone. Erosion may be a result of acidic foods, drinks, and acidic medications. However, the most common cause is gastroesophageal reflux disease (GERD) that can result in severe tooth erosion. Reflux occurs when the lower esophageal sphincter relaxes, resulting in a decrease in the pressure gradient between the esophagus and stomach. The refluxed material may enter the oral cavity and result in erosion. Possible causes of GERD include obesity, hiatal hernia, and pregnancy. Recumbency, lifting heavy objects, or bending after a meal sometimes causes food or liquid to rise up into the throat. Treatment of GERD may involve changes in diet, amounts of food consumed, and prescribing antisecretory medications.4
Anorexia and bulimia nervosa are eating disorders resulting from psychological manifestations. Anorexia is characterized by a fear of becoming fat. This results in food evasion, reduced body weight, amenorrhea, and vomiting or purging. Bulimic patients consume food in large quantities because of amplified hunger and then induce vomiting to expel the recently ingested food. Initially the palatal surfaces of maxillary anterior teeth are affected, then the occlusal surfaces of the teeth in both arches, and then the labial or buccal surfaces are affected after a longer period of time. The lingual surface of lower anterior teeth are usually the last to exhibit dental erosion. The increased erosion will result in sensitivity. In addition, many of these patients drink excessive amounts of sweetened carbonated drinks that contribute to loss of tooth structure from erosion.5
Acid erosion destroys tooth structure by first disintegrating enamel and then the dentin. Initially, the enamel prism is dissolved and then the less mineralized dentin is eroded more expeditiously. The acid enlarges the dentinal tubules and then progresses into the intertubular areas, thus resulting in a rough and porous surface. Eventually this process continues until the pulp is involved. Sclerotic dentin may result from mild but long-standing acid attack. This is a result of mineral salt deposition in the intratubular dentin. The organic component is reduced and the peritubular dentin width will increase as a result of the mineral salts that are deposited in the intertubular dentin. The resulting decrease in the organic section will decrease the effectiveness of dentin bonding agents. Gwinnett and Jendresen demonstrated that resin tag formation in sclerotic dentin was greatly reduced.6 The adhesion between the sclerotic dentin and the bonding agent consists of resin impregnation into the demineralized intertubular dentin.
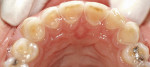
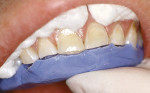
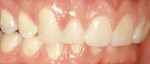
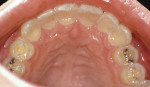
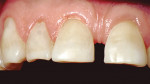
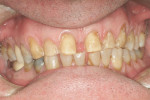
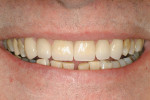
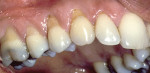
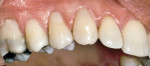
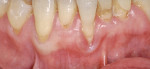
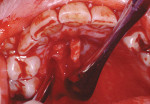
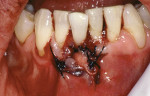
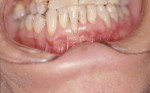
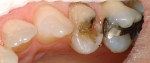
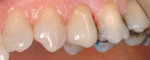
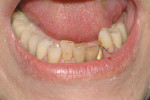
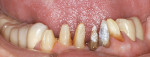
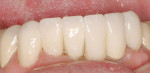
Treatment can be minimally invasive with dentin bonding and composite, as accomplished for a patient whose chief complaint was tooth sensitivity and lack of tooth display affecting his social life (Figure 1; Figure 2; Figure 3; Figure 4). He was made aware that this was an interim treatment until the causes could be eliminated and more comprehensive treatment could be accomplished. His school schedule only permitted palliative treatment. Porcelain veneers or full-coverage crowns were other options; a long-term solution was what this patient needed. The amount of tooth structure lost and the desire for esthetics and/or longevity would dictate appropriate treatment.
In the early 1980s, McCoy stated that tooth flexure from tensile stresses led to cervical breakdown and created angled notches.7 Abfraction is a term coined by Grippo that describes a loss of hard tissue at the cementoenamel junction (CEJ) caused by biomechanical effects of occlusal loading as suggested by McCoy.8 Abfraction lesions are angular, wedge-shaped defects at the cervical area. Occlusal forces create stress throughout tooth structure. These forces can be caused by normal function or parafunction. The biomechanical theory states that the mechanical overloading of cervical enamel from cuspal flexure may cause noncarious cervical loss of tooth structure.9 Flexure of the tooth causes tensile and shear stresses in the cervical area, resulting in a disruption of the bonds between the hydroxyapatite crystals and possibly forming cracks. Although dentin is stronger than enamel under lateral forces, this cyclic compression and tension may result in the split of the chemical bonds between the hydroxyapatite crystals.10 The enamel in this area also has incremental lines caused by changes in crystal orientation and reduced crystal diameter. These lines may serve to cause cleavage, as they coincide with the plane of highest stress. The lower periodontal area of maxillary incisors and premolars, compared to the maxillary canine, are less likely to withstand occlusal and parafunctional loads. The labial plate around the maxillary incisors is also thin compared to the canine and premolar; therefore, a greater displacement will occur under occlusal forces.11 Although occlusal loading may initiate this process, the progression of the lesion may be caused by other factors.
Loss of tooth structure in the cervical area from these noncarious lesions may present esthetic problems and may be a source of hypersensitivity.10 The sensitivity is both physical (cold, air, tensile) and hyperosmotic (glucose solution). This is associated with the patency of the dentinal tubules. Treatment of these lesions can be done restoratively, with composites, glass ionomers, porcelain veneers, and crowns, or by periodontal grafting. Careful evaluation is needed to determine whether a single or combined treatment will provide the best long-term results. Erosion, abrasion, or abfraction lesions that do not extend onto the root surface should be treated restoratively. Class III recessions with cervical tooth wear are better off with a combination of surgical and restorative treatment.12 The appropriate treatment will be guided by esthetic considerations and then modified by the outcome of the patient consultation and existing bone levels. In the author’s opinion, if the patient has a high smile line and the teeth without recession are the correct length, shape, and shade, periodontal plastic surgery would be the preferred technique to eliminate sensitivity and improve appearance. If the patient requires changes in shape and shade, grafting in addition to restoration with veneers or crowns should be considered. If the teeth are too short and the incisal edge needs lengthening, the restorative option with veneers or crowns will improve the esthetics and cover any gingival defects to eliminate sensitivity.
Figure 5; Figure 6; Figure 7 show a patient who had previous composite bonding covering the gingival aspect of his anterior teeth. The restorations eventually discolored and he also wanted to change the shape, spacing, and color of his teeth. He elected to have porcelain veneers placed on his six maxillary anterior teeth initially and then on his premolars subsequently. Figure 8 and Figure 9 show another patient who had minimal recession. In patients with minimal recession who do not want or require grafting or veneers, a class V restoration with composite will eliminate sensitivity and reduce the chance of gingival caries.
Grafting is another option and can be done by a variety of techniques.13 Even if the patient had a previous restoration, that restoration could be removed and the defect reshaped by diamond burs and curettes to eliminate sharp edges, reduce the mesiodistal dimension of the dentin to be covered, and create a concave defect.14 If the patient has a high smile line and placing a restoration will create teeth that are too long, grafting would be the preferred technique. The reluctance of patients to do a surgical procedure may sometimes be overcome by the use of an allograft. Acellular dermal matrix allograft is especially useful in multiple sites.15 However, when a defect is circumferential, root coverage is not predictable and the height of the interproximal bone will dictate the amount of facial coverage that can be accomplished.
In Figure 10; Figure 11; Figure 12; Figure 13, the patient had an area of recession on a lower anterior tooth that was getting progressively worse and was sensitive but the amount of coverage was limited by the adjacent interproximal bone.16 Facial recession alone can be successfully treated with free gingival grafts, connective tissue grafts, laterally positioned flaps, and guided tissue regeneration.17,18 The higher the Miller classification, the less favorable the prognosis.17
The term cracked tooth was initially described by Richey et al and Cameron as an incomplete fracture of a vital posterior tooth that involves dentin and may extend to the pulp.19,20 The most common causes are related to cavity preparation, such as deep and extensive cavity preparations, occlusal and parafunctional causes, and trauma. Cracks also can occur in teeth without any restorations in developmental fissures, and occlusal interferences may produce stresses that exceed the elastic limit of dentin.21 Cracked teeth usually exhibit pain during chewing or biting. The pain is usually expressed when pressure on a specific part of the tooth is released. The pain created upon the release of pressure is caused by fluid in the crack moving toward the pulp. In vital teeth, additional symptoms that may present are sensitivity to a change in temperature, especially colder temperatures. The hypersensitivity may also be a result of a mild pulpitis caused by bacteria in the cracks.
Diagnosis is aided by transillumination and staining the tooth with methylene blue or a similar agent. Sometimes the crack can only be visualized by removing the current restoration. Usually percussing the affected area of the tooth will elicit pain. Extreme pain can be caused by pressure applied on an individual cusp. However, once the force is removed, the pain will usually go away. The tooth is not normally sensitive in an axial direction and no radiolucent area is present unless the fracture extends into the pulp. Treatment will depend on the severity of the symptoms and the location of the crack. If the crack has not progressed too far, protection of the cusps with an onlay or full-coverage crown will eliminate symptoms. A bonded restoration with or without cuspal coverage may prevent the cusps from fracturing. If a fracture does occur, the missing portion can be replaced with the bonded restoration and help to reinforce the remaining tooth structure.
In Figure 14 and Figure 15, the patient had complained of sensitivity to biting and cold on tooth No. 12. She had a history of previous cusps fracturing and the teeth have been restored with onlays. Upon removal of the distal-occlusal amalgam in tooth No. 12, the buccal cusp was seen to be fracturing. Because of the difficulty in blending the margin of an onlay in this area and an abfraction lesion at the gingival margin, it was elected to place a porcelain onlay-veneer restoration. If the remaining tooth structure was weakened or undermined, in the author’s opinion it would be prudent to onlay the remaining cusps. 22 The long-term bond of the cracked tooth structure to the restoration is questionable, and some clinicians will remove or onlay tooth structure exhibiting a crack.19 However, if the crack extends into the pulp, endodontic treatment and a crown may be necessary. More complex cracks and deeply cracked teeth may not respond to treatment and may need extraction.
Preparation of teeth for porcelain veneers or crowns may also lead to sensitivity for several reasons. 23 This is especially true for mandibular incisors because of their limited coronal dimensions. It has been recommended in recent years to align teeth by so-called “instant orthodontics.” However, the degree of misalignment may affect the success of the restorations. The patient seen in Figure 16 ; Figure 17 ; Figure 18 wanted to improve the appearance of her mandibular teeth. She also wanted to minimize full coverage with crowns. However, to align the incisal edges and eliminate old proximal restorations and the overlap of the teeth, aggressive preparations were required. Orthodontics would usually be preferred but, because of the patient’s age and existing porcelain-fused-to-metal bridges, this option was not feasible. Ideally, no exposure of dentin or minimal exposure is ideal. The preparation should ideally end in enamel because the bond to dentin is weaker and more technique-sensitive. In addition, esthetics may be compromised as gingival margins and papillae cannot be aligned appropriately.24
Permanent restorations do not irritate the pulp but any bacteria present in the smear layer or deep in the tubules may lead to infection. A provisional restoration that does not seal the margins may allow the ingress of bacteria. Heat build-up during preparation may damage the pulp because stasis and thrombosis may develop in pulpal blood vessels. Drying the dentin by air may cause irritation and dislocation of odontoblasts and erythrocytes into the dentinal tubules. As these cells degenerate, an inflammatory response can occur in the pulp.25
Full-crown preparations cause more pulpal inflammation than partial preparations. The closer the preparation comes to the pulp, the diameter and amount of the dentinal tubules increases. This will increase the chance of getting bacteria into the pulp. In the last few years it has been suggested to seal the dentin surface immediately after preparation. It is believed that dentin sealing will protect the dentin against bacterial invasion during provisionalization. This may be even more significant for porcelain veneer preparations, as a seal is not usually obtained with the provisional restorations.26,27
Bleaching is a conservative technique of improving the esthetic appearance of teeth. However, possible side effects such as sensitivity have to be taken into consideration. Before initiating treatment, any potential causes of increased dentin permeability—such as acidic food or beverages, acid reflux, toothbrush abrasion and resulting recession—have to be considered. To reduce sensitivity, occluding the dentinal tubules or using materials that block nerve conduction is recommended. Materials such as potassium nitrate are sometimes used as a dentifrice or in a tray 2 weeks before bleaching to minimize any sensitivity. The potassium nitrate also can be used once sensitivity occurs. The potassium nitrate prevents nerve repolarization after depolarization and this prevents the nerve from transmitting pain signals.
Other materials work to block patent dentinal tubules. Their mode of action is limited to reducing osmotic stimulation as they do not prevent peroxide diffusion into the pulp.28 Using materials containing amorphous calcium phosphate serves as a reservoir for calcium and phosphate that remain available in the saliva for several hours after initial use. Some whitening materials contain this as an additive and have been shown to reduce sensitivity.29
The sensitivity that may result from bleaching has several possible causes. The key ingredients in home-whitening gels are carbamide peroxide or hydrogen peroxide, glycerin or propylene glycol as the carrier, and carbopol as a thickening agent. Phosphoric acid or citric acid may also be added to reduce the pH to increase the shelf life of the product. The carbamide peroxide forms hydrogen peroxide and urea. The hydrogen peroxide, which causes the whitening, penetrates into the tooth structure and forms oxygen and water. The oxygenation of pigment molecules results in whitening. The penetration of the peroxide into pulp occurs in 5 to 15 minutes resulting in irritation to the nerves and a reversible pulpitis.28 However, pulpal irritation usually resolves within 2 weeks after treatment has been completed.28 Even long-term treatment for tetracycline-stained teeth does not result in prolonged sensitivity.30
The use of hydrogen peroxide whitening gels may also result in increased tooth sensitivity. Because hydrogen peroxide releases all of its oxygen in 30 to 60 minutes, it quickly penetrates into pulpal tissue and may cause more sensitivity compared to carbamide peroxide.28
Conclusion
A thorough evaluation of a patient should include a past history of any sensitivity. If any sensitivity exists, the variety of techniques available should be discussed with the patient along with the benefits and disadvantages. A variety of materials are available for desensitizing but the effect is transitional and the dentist or the patient have to reapply periodically. If the patient desires esthetic procedures, the possibility of subsequent sensitivity that may occur should be addressed. There are many causes of sensitivity and ideally the cause/or causes should be determined before commencing treatment to eliminate the problem. When new treatment is initiated, care should be taken to minimize any possible iatrogenic sensitivity problems. Proper attention to detail will minimize any untoward problems, but if they occur, the treating dentist must know how to address them.
References
1. Rimondini L, Baroni C, Carrussi A. Ultrastructure of hypersensitive and non-sensitive dentine. A study on replica models. J Clin Periodontol. 1995;22(12): 899-902.
2. Bamise CT, Olusile AO, Oginni AO. An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract. 2008;9(5):52-59.
3. Jacobsen PL, Bruce G. Clinical dentin hypersensitivity: Understanding the causes and prescribing treatment. J Contemp Dent Pract. 2001;2(1): 1-12.
4. Van Roekel NB. Gastroesophageal reflux disease, tooth erosion, and prosthodontic rehabilitation: A clinical report. J Prosthet Dent. 2003;12(4): 255-259.
5. Kavoura V, Kourtis SG, Zoidis P, et al. Full-mouth rehabilitation of a patient with bulimia nervosa. A case report. Quintessence Int. 2005;36(7-8):501-510.
6. Gwinnett AJ, Jendresen MD. Morphological features of cervical erosion after acid conditioning and its relation with composite resin. J Dent Res. 1978;57(4):543-549.
7. McCoy G. On the longevity of teeth. J Oral Implantol. 1983;11(2):248-267.
8. Grippo JO. Abfractions: a new classification of hard tissue lesions of teeth. J Esthet Dent. 1991;3(1):14-19.
9. Rees JS, Jacobsen PH. The effect of cuspal flexure on a buccal Class V restoration: a finite element study. J Dent. 1998;26(4): 361-367.
10. Pegoraro LF, Scolaro JM, Conti PC, et al. Noncarious cervical lesions in adults: prevalence and occlusal aspects. J Am Dent Assoc. 2005: 136(12):1694-1700.
11. Rees JS, Hammadeh M, Jagger DC. Abfraction lesion formation in maxillary incisors, canines and premolars: A finite element study. Eur J Oral Sci. 2003;111(2): 149-154.
12. Toffenetti F, Vanini L, Tammaro S. Gingival recessions and noncarious cervical lesions: A soft and hard tissue challenge. J Esthet Dent. 1998;10(4):208-220.
13. Camargo PM, Lagos RA, Lekovic V, Wolinsky LE. Soft tissue coverage as treatment for cervical abrasion and caries. Gen Dent. 2001;49(3):299-404.
14. Prato GP, Tinti C, Cortellini P, et al. Periodontal regenerative therapy with coverage of previously restored root surfaces: case reports. Int J Perio Rest Dent. 1992; 12(6):450-461.
15. Henderson RD, Greenwell H, Drisko C, et al. Predictable multiple site root coverage using an acellular dermal allograft. J Periodontol. 2001;72(5):571-582.
16. Miller PD Jr. A classification of marginal tissue recession. Int J Perio Rest Dent. 1985; 5(2):8-13.
17. Rossberg M, Eickholz P, Raetzke P, Ratka-Krüger P. Int J Perio Rest Dent. 2008; 28(1):19-27.
18. Kassab MM, Cohen RE. Treatment of gingival recession. J Am Dent Assoc. 2002; 133(11):1499-1506.
19. Ritchey B, Mendenhall R, Orban B. Pulpitis resulting from incomplete root fracture. Oral Surg Oral Med Oral Oral Med Oral Pathol. 1957;10:665-670.
20. Cameron CE. Cracked tooth syndrome. J Am Dent Assoc. 1964;68:405-411.
21. Signore A, Benedicenti S, Covani U, Ravera G. A 4- to 6-year retrospective clinical study of cracked teeth restored with bonded indirect resin composite onlays. Int J Prosthodont. 2007;20(6): 609-616.
22. Geurtsen W, García-Godoy F. Bonded restorations for the prevention and treatment of the cracked-tooth syndrome. Am J Dent. 1999;12(6): 266-270.
23. Cherukara GP, Davis GR, Seymour KG, et al. Dentin exposure in tooth preparation for porcelain veneers: a pilot study. J Prosthet Dent. 2005;84(5):414-420.
24. Jacobson N, Frank CA. The myth of instant orthodontics: an ethical quandary. J Am Dent Assoc. 2008;139(4): 424-434.
25. Bränström M. Reducing the risk of sensitivity and pulpal complications after the placement of crowns and fixed partial dentures. Quintessence Int. 1996;27(10):673-678.
26. Magne P. Immediate dentin sealing: a fundamental procedure for indirect bonded restorations. J Esthet Restor Dent. 2005; 17(3):144-154.
27. Magne P, So WS, Cascione D. Immediate dentin sealing supports delayed restoration placement. J Prosthet Dent. 2007; 98(3):166-174.
28. Hewlett ER. Etiology and management of whitening-induced tooth hypersensitivity. J Calif Dent Assoc. 2007;35(7): 499-506.
29. Ginger M, Spaid M, et al. A 180-day clinical investigation of the whitening efficacy of bleaching gel with added amorphous calcium phosphate. J Clin Dent. 2005;16:11-16.
30. Matis BA, Wang Y, Eckert GJ, Cochran MA, Jiang T. Extended bleaching of tetracycline-stained teeth: a 5-year study. Oper Dent. 2006;31(6):643-651.
About the Author
Richard Trushkowsky, DDS
Associate Professor
New York University College of Dentistry
New York, New York
Private Practice
Staten Island, New York