Nano-Ionomer Restorative Cement: Observations After 2 Years of Use
Theodore P. Croll, DDS; and Joel H. Berg, DDS, MS
Glass-polyakenoate (ionomer) systems are the best direct-application dentin replacement materials available for the dental clinician. Of these, the resin-modified glass-ionomers (RMGIs) have the best physical characteristics, in addition to excellent handling properties and all advantages germane to glass ionomers. Their ability to harden rapidly when exposed to a visible light beam make them the most practical to use. Since their introduction in the late 1980s, they have become a mainstay material for repairing teeth, cementing orthodontic bands, and luting various types of full-crown restorations in both primary and permanent teeth.
Likewise, resin-based composites (RBCs) are the best adhesively bonded direct-application enamel replacement material. With varying particle types, sizes, and distributions, RBCs have been made to replicate the strength, wear resistance, shades, textures, and polishability rivaling the enamel they were designed to replace.
Based on the principles of biomimesis (a bioengineering concept and treatment goal, whereby biologic components are designed to replicate natural essence as closely as possible1,2), many dentists have used RMGI and RBC in concert for tooth repair. After placement of RMGI cement for dentin restoration, RBC is overlaid for enamel replacement. This method of tissue-specific tooth repair, analogous to the natural layering and intimate biologic connection of dentin and enamel, has been called "the sandwich technique," "lamination," and restorative "stratification."2-6
Berg described the continuum of adhesive tooth-restorative materials available to modern dentists.7 Every point on the continuum marks a spot for a repair material with positive features and accompanying negative attributes. Dental materials scientists know that dentists would appreciate having one dental repair material that incorporates all the advantages of both hydrophilic RMGIs and moisture-resisting RBCs, while eliminating the disadvantages of each. Such a material would:
- chemically bond to enamel and dentin.
- be therapeutic by releasing fluoride ions that are incorporated into adjacent dentin and enamel, rendering that tooth structure less soluble to acid challenge.
- have an antimicrobial effect by virtue of its fluoride content.
- have an equivalent coefficient of thermal expansion to that of tooth structure so that the mass of material had sufficient dimensional stability, minimizing marginal breakdown.
- not shrink or expand during the hardening reaction.
- be insoluble in oral fluids and foodstuffs (erosion resistance).
- have high resistance to wear from impact forces and stresses from occlusion and mastication (and wear and tear from toothbrushing).
- have high cohesive strength and resistance to both initial fracturing and propagation of fractures.
- be tooth-colored, highly polishable, and easy to handle, including an on-command hardening characteristic (such as photopolymerization).
In 2007, a novel RMGI was introduced. Ketac™ Nano (3M ESPE, St. Paul, MN) is marketed as the first "nano-ionomer." Unlike Vitremer™ (3M ESPE) which is a RMGI restorative cement supplied in a powder-liquid format, Ketac Nano is offered in a double-barreled “clicker" assembly. This storage/delivery system exudes desired portions of two pastes simultaneously for hand spatulation on a mixing pad. The blended paste is then ready for syringe injection into a cavity preparation. After more than 2 years of using the nano-ionomer (some premarket use included), the authors believe that this formulation represents a major step in the quest to develop an ideal direct-application adhesive dental restorative material combining the positive features of RMGIs and RBCs. In the manufacturer’s technical product profile, the nano-ionomer is described:
"Paste A is resin based and contains fluoroaluminosilicate glass, silane-treated silica and zirconia silica nanofillers, methacrylate and dimethacrylate resins, and photoinitiators. Paste B is water based and contains polyalkenoic acid copolymer (Vitrebond™copolymer, 3M ESPE), silane-treated zirconia silica nanoclusters, silane-treated silica nanofiller, and hydroxymethylmethacrylate (HEMA). Ketac™ Nano Primer contains water, HEMA, polyalkenoic acid copolymer, and photoinitiators.”
The nanofiller (5 nm to 25 nm) and nanofiller clusters (1 µm to 1.6 µm) are “loosely bound agglomerates of nanosized zirconia/silica" that compose approximately 60% of the glass component of Ketac Nano and make for higher filling loading, according to the 3M ESPE technical product profile. The manufacturer also reports that this radiopaque restorative material has fluoride ion fluid dynamics, like other glass ionomers, as well as higher strengths, enhanced esthetics, and better polishability than other RMGIs.
Indications
Indications for Ketac Nano, reported in the manufacturer's product profile are:
- Primary teeth restorations
- Small class I restorations
- Class III and class V restorations
- Transitional restorations
- Filling defects and undercuts
- Laminate/sandwich technique
- Core build-ups where at least 50% of coronal tooth structure remains for support
Technique
Ketac Nano is used as follows:
- Ketac Nano Primer is painted within the cavity preparation and over the cavosurface margins. The primer thoroughly wets the cavity preparation, making for intimate chemical contact at tooth-cement interfaces while solubilizing the smear layer. Excess primer can be blotted with the microapplicator or thinned with a soft stream of dry air.
- The curing light beam is applied for 10 seconds.
- The material is "clicked out" onto a mixing pad and blended rapidly and completely with a spatula. One click usually suffices for small restorations. Thorough spatulation for approximately 20 seconds is essential for complete blending of components.
- The material then is scooped up in a thin-lumen AccuDose® orange syringe tip (Centrix, Inc, Shelton, CT). At this time, it is helpful to let the material congeal in the syringe tip for 30 to 40 seconds. This delay gives the blend some body and makes for easier placement and less stickiness.
- Then, the material is injected slowly within the internal aspects of the preparation, without entrapping air bubbles.
- According to the manufacturer, increments no thicker than 2 mm should be placed and light-cured individually. The authors have found that 8 seconds of light application (1,100 mW/cm2), hardens each layer. (Other RMGIs have three hardening reactions; photocuring, chemical resin curing, and the glass-ionomer acid-base reaction). Ketac Nano does not have a chemical resin curing component, so complete light-beam saturation is important.
- The nano-ionomer should overfill the preparation, with the last layer compressed on the tooth surface to ensure complete fill at all margins. The authors use a large ball burnisher for occlusal restorations and a wide flat instrument on axial surface restorations for that purpose. The instrument is dipped in Ketac Nano Primer so that the material does not stick and pull away. After complete light-hardening of the final layer, anatomical form is sculpted using slow-speed diamond burs.
- Finishing, polishing, and occlusal adjustments proceed in the same manner used to complete a RBC restoration (slow-speed medium and fine diamond burs, aluminum oxide or diamond finishing strips and discs). In some cases, after finishing is completed, the authors have sealed the treated surface using a self-etching bonding agent and clear resin sealant, using the method of Croll and Donly.7 Some restored surfaces also have been refinished and sealed at routine reevaluation appointments, using the same method.
Case Examples
The authors have used the nano-ionomer in its current marketed formulation and in a preproduction formulation since early 2006. The following cases typify results they have observed at 10 months to 2 years after treatment.
Case 1
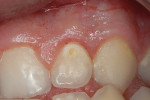
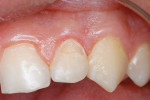
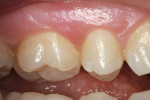
A teenaged boy had a decalcification/caries lesion of the lateral incisor (Figure 1a). Class V preparation included mechanical undercutting retention form (Figure 1b). After 12 months, the repair was imperceptible (Figure 1c).
Case 2
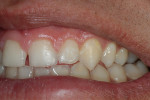
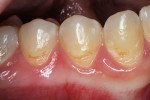
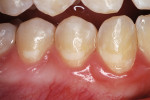
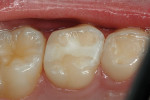
A teenaged girl had poor oral hygiene during orthodontic therapy and developed smooth surface caries lesions (Figure 2a). The nano-ionomer restorations were intact 10 months after treatment (Figure 2b). The shade match was not ideal, but the repaired areas were well below the lip line and imperceptible.
Case 3
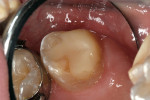
A teenaged girl had a mesiobuccal “slot” repair8 using a nano-ionomer. The restoration conserved the marginal ridge and was intact, 14 months after placement (Figure 3a and Figure 3b).
Case 4
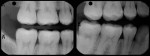
A 6-year-old girl exhibited a large intracoronal lesion soon after eruption of her permanent first molar (Figure 4a). Because the caries infection nearly exposed the dental pulp, caries control was completed using a calcium hydroxide liner (Dycal®, DENTSPLY Caulk, Milford, DE) (Figure 4b), a RMGI base (Vitrebond) (Figure 4c), and Ketac Nano. After 13 months, the restoration was intact and the tooth had no clinical or radiographic signs of pulpal pathologic changes (Figure 4d). At that time, the occlusal surface was roughened and sealed with a self-etching bonding agent and clear resin sealant9 (Figure 4e).
Case 5
A 10-year-old girl had an enamel hypoplasia defect with associated dental caries (Figure 5a). The occlusolingual cavity preparation included all of the hypoplastic and carious tooth structure. An RMGI liner was placed before the nano-ionomer was injected.10 The restoration was intact 16 months after treatment (Figure 5b).
Case 6
This 10-year-old patient had an even larger enamel hypoplastic defect than seen in the patient in Case 5.10 The occlusobuccodistolingual interim repair (with RMGI liner) was intact, 14 months after treatment (Figure 6a and Figure 6b).
Case 7
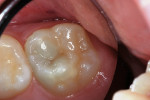
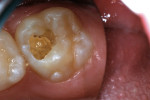
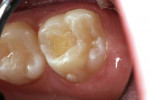
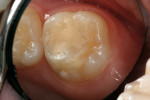
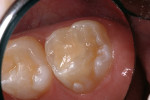
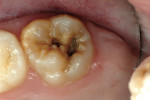
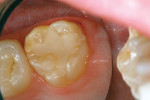
A 9-year-old patient had distal surface caries of the mandibular primary second molar and caries lesions of the mesial and distal surfaces of the maxillary primary second molar. Class II nano-ionomer restorations of both teeth were in-tact, 12 months after treatment (Figure 7a, Figure 7b, and Figure 7c).
Discussion
The nano-ionomer is easy to use after the clinician gains a little experience. A properly blended mixture will be sticky and difficult to manipulate unless it is syringe-injected into place. The authors strongly recommend the smallest lumen AccuDose orange syringe tip. Pinpoint placement and slow injection is important, and the wider lumen tips make for less precise placement. Leaving the blended material within the syringe tip for a short time after mixing will make for slight cement solidification, improving handling during injection.
In some cases, color matching for permanent anterior tooth repair can be difficult. The color may match well at initial placement, but the hardened material subsequently lightens in shade (see Case 2). For the most part, the authors have solved this problem by overcompensation (using a slightly darker shade of material at initial placement). As soon as the material is light-hardened, the shade difference seems to disappear, or at least improve noticeably. Fortunately, the glass-filled material refracts and reflects light in such a manner that the optical effects of surrounding tooth color make for imperceptible interfaces. The polishability of the nano-ionomer and effect of salivary moistening also combine to camouflage the restorative material.
Although the manufacturer recommends cement curing in 2-mm increments, the authors have bulk-cured class II restorations of primary molars using a visible light beam of 1,100 mW/cm2, applied for an initial 16 seconds with an additional 8-second exposure from the occlusal aspect, after all finishing and occlusal adjustment was completed. Their testing with extracted primary teeth in the laboratory and their clinical observations over 2 years have confirmed sufficient through-and-through hardening using that protocol.11 Curing lights having less power will require different exposure protocols.
In larger preparations, the authors recommend routinely lining the cavity preparations with a layer of RMGI liner/base material, as described in Case 4. The liner ensures complete internal coverage of all exposed dentin, virtually eliminates postoperative tooth sensitivity, and decreases the distance needed for light penetration. In Case 4, the Vitrebond base, with its antimicrobial effect, fluoride ion content of the reactive glass filler, and chemical bond to dentin gives the pulp an opportunity to heal without further bacterial insult. The overlying nano-ionomer bonds to the tooth structure, along with the liner. This combination serves as an interim enamel replacement with much better physical properties and longevity than the reinforced zinc-oxide/eugenol temporary filling, traditionally used for such caries-control purposes. The authors also have restored many hypoplastic permanent molars, carious or not, with the nano-ionomer.10 An RMGI base is used routinely in deeper preparations to repair such teeth (see Case 5 and Case 6).
With regard to the long-term durability of nano-ionomer restorative material, the authors have found that many conservative class I glass-ionomer silver-cermet restorations have lasted well into a second decade with minimal wear or erosion and no need for resurfacing. Further, occlusal surface RMGI “interim” restorations have routinely lasted 10 years or more before resurfacing or replacement has been required. RMGIs used for smooth surface repairs, not undergoing impact forces and stresses of occlusion and mastication, have proven durable and reliable for many years after placement since their introduction in the early 1990s. When one considers the history of these glass-ionomer systems that have proven themselves and the nano-ionomer’s enhanced physical properties, it is logical to assume that the nano-ionomer should hold up well in the mouth, as well. It is also reasonable to assume that dental manufacturers already are working on improvements in glass-ionomer nanotechnology that will progress even further in the quest for an ideal direct-application dental repair material.
References
1. Bugliarello G. Biomimesis: the road less traveled. The Bridge. 1997;27(3):2-3.
2. Croll TP, Cavanaugh RR. Posterior resin-based composite restorations: a second opinion. J Esthet Restor Dent. 2002;14:(5)303-312.
3. McLean JW, Wilson AD. The clinical development of the glass-ionomer cement. II. Some clinical applications. Aust Dent J. 1977;22(2):120-127.
4. McLean JW, Powis DR, Prosser HJ, et al. The use of glass-ionomer cements in bonding composite resins to dentine. Br Dent J. 1985;158(11):410-414.
5. Wilson AD, Mc Lean JW. Laminate restorations. In: Glass-Ionomer Cement. Chicago, IL: Quintessence Publishing Co; 1988:159-178.
6. Mount GJ. Clinical requirements for a successful "sandwich"- dentine to glass-ionomer cement to composite resin. Aust Dent J. 1989;34(3):259-265.
7. Berg JH. The continuum of restorative materials in pediatric dentistry—a review for the clinician. Pediatr Dent. 1998;20(2):93-100.
8. Croll TP. Lateral-access Class II restoration using resin-modified glass-ionomer or silver-cermet cement. Quintessence Int. 1995;26(2):121-126.
9. Croll TP, Donly KJ. Resin-based composite margin repair. Inside Dentistry. 2008;4(6):92-93.
10. Croll TP. Restorative options for malformed permanent molars in children. Compend Contin Educ Dent. 2000;21(5):676-682.
11. Croll TP, Berg JH. Resin-modified glass-ionomer restoration of primary molars with proximating Class II caries lesions. Compend Cont Educ Dent. 2007;28(7): 372-377.
About the Authors
Theodore P. Croll, DDS
Private Practice in Pediatric Dentistry
Doylestown, Pennsylvania
Affiliate Professor
Department of Pediatric Dentistry
University of Washington School of Dentistry
Seattle, Washington
Adjunct Professor
Pediatric Dentistry
University of Texas Health Science Center at San Antonio (Dental School)
San Antonio, Texas
Joel H. Berg, DDS, MS
Professor and Chair
Department of Pediatric Dentistry
University of Washington School of Dentistry
Seattle, Washington