New Techniques and Technology in Implant Dentistry Reduce Treatment Time
Robert Emery, DDS; Benjamin Watkins; Steven A. Guttenberg
Implant dentistry continues to evolve, and biomedical scientists and engineers are exploring new concepts with advanced technologies. Treatment has evolved from the days of high-speed intraoral preparation with a conventional dental handpiece (blade implants) to the first days of the two-stage implant protocol, using machined-surfaced implants (unloaded healing) with two surgeries in edentulous patients.
Today, patients missing one or more teeth have come to expect excellent, natural esthetics along with a return to normal function in an accelerated timeline. Dental implants have evolved from commercially pure titanium-machined surfaces to enhanced surfaces such as those with hydroxy-apatite coatings, or acid-etched surfaces. For example, with OSSEOTITE® Implants (BIOMET 3i, Palm Beach Gardens, FL), long-term cumulative survival rates improved from the 85% to 95%1,2 seen with machined implants to the 95% to 98%3,4 range, reported by numerous researchers around the world using the OSSEOTITE surface.
Implants typically demonstrate good primary stability at the time of placement—in principle, a mechanical phenomenon. However, when bone remodels in the weeks after implant placement, primary implant stability can degrade, which in turn might impact the ability to perform early or immediate loading protocols. This remodeling may lead to an increased potential for implant failure.
In the continued evolution of biomedical science, a new world has been discovered in the form of nanotechnology. To this end, dental researchers have added nanotechnology to dental implant surfaces. For instance, nano-scale crystals of calcium phosphate have been placed onto BIOMET 3i’s proprietary OSSEOTITE surface by using a Discrete Crystalline Deposition™ (DCD™) process. This process of applying nanotechnology resulted in a new surface that leverages the OSSEOTITE surface5 as the substrate while maximizing the known biologic benefits of calcium phosphate in bone formation and healing. The result: the NanoTite™ Implant, an implant with a more complex topography that may have the potential to accelerate healing. In the end, the intent is to provide implant therapy to more patients with higher survival rates and shorter overall treatment times.
The clinical case presentation demonstrates an accelerated treatment protocol with new technology and includes cone beam computed tomography, a sinus lift, immediate implant placement, and immediate provisionalization of implants in the posterior maxilla.
CASE PRESENTATION
The posterior maxillae present unique anatomic challenges to the implant team. Poor bone quality and limited volume secondary to sinus pneumatization of the maxillary alveolus can lead to the need for surgical site preparation before implant placement. Esthetic and functional considerations of maxillae often make removable provisional restorations impracti-cal. New enhanced implant surfaces may provide the implant team with the confidence to immediately load implants in compromised sites.
Initial Patient Presentation
A 55-year-old male was referred for reconstruction of his posterior maxilla. His chief complaint was, “I must be able to speak and eat in front of my patrons.” He also did not want any type of removable prosthetic replacement for the missing teeth. Radiographic and clinical examination revealed acute, localized bone loss around tooth No. 3, a radiolucency on tooth No. 5, and significant mobility of the existing fixed partial denture (FPD) on teeth Nos. 4 through 7 (Figure 1 and Figure 2).
Diagnosis
The diagnosis included:
- Class II malocclusion, without dysfunction;
- moderate occlusal abrasion secondary to parafunctional habits;
- recurrent dental caries on teeth Nos. 4 and 5 (maxillary right premolars);
- a non-restorable FPD on teeth Nos. 4 through 7;
- inadequate bone volume for implant placement, maxillary right cuspid;
- severe localized periodontitis on tooth No. 3 (maxillary right first molar);
- significant maxillary right sinus pneumatization; and
- failing endodontic therapy on tooth No. 5.
Treatment Plan
The treatment plan included:
- diagnostic casts, wax patterns, and fabrication of a laboratory-processed fixed provisional restoration for teeth Nos. 2 through 7;
- extraction of teeth Nos. 3 and 5, alveolar preservation grafts, and ridge expansion on tooth No. 6;
- placement of a provisional FPD on teeth Nos. 2 through 7;
- healing of osseous defects, maxillary right posterior quadrant;
- extraction and immediate implant placement of tooth No. 4, and placement of implants in tooth positions Nos. 3, 5, and 6;
- placement of CAD/CAM healing abutments and definitive impression;
- immediate loading with a screw-retained provisional restoration; and
- placement of definitive abutments and prosthesis.
Surgical Treatment
This case required maintenance of a tooth with a hopeless prognosis (No. 4) for use as a provisional bridge abutment while the future implant sites underwent osseous healing after extraction, grafting, and ridge expansion. The extractions were done in sequence: teeth Nos. 3 and 5 were extracted in conjunction with grafting and ridge splitting in the areas of teeth Nos. 5 and 6, respectively. A provisional FPD was made, using teeth Nos. 2, 4, and 7 as the posterior and anterior abutments. To minimize chairtime and future maintenance issues, a laboratory-fabricated provisional restoration with metal reinforcement was fabricated by the restorative dentist before surgery.
Teeth Nos. 3 and 5 were extracted. A Piezosurgery® device (Piezosurgery, Inc, Matawan, NJ) was used to split the ridge and the palatal alveolus was expanded. The expansion was maintained with a 1.5-mm lag screw. All sites were grafted with mineralized cancellous allograft. In site No. 6, a slowly resorbable barrier collagen membrane was placed and primary closure was achieved. In site No. 3, additional fixed, keratinized, attached tissue was desired and a perforated 100% polytetrafluoroethylene (PTFE) membrane was used. The prefabricated provisional FPD was placed and the patient was allowed to function for the 4 months of bone maturation.
Four months after the tooth extraction and grafting, the patient returned for the second surgical phase of treatment: implant placement and implant provisional restoration. The fixed provisional restoration was removed. Tooth No. 4 was extracted. A small incision was made to remove the lag screw. The surgical guide was indexed to the anterior tooth preparation No. 7. NanoTite PREVAIL® Implants (BIOMET 3i) were placed into teeth Nos. 3 through 6, with a sinus elevation performed in site No. 3. Composite bone grafts were used to augment the sites. Insertion torque values greater than 35 Ncm were obtained in all sites except tooth No. 3, which had an insertion torque value of 20 Ncm. The surgeon placed Encode® Healing Abutments (BIOMET 3i) with emergence profiles consistent with the teeth being replaced (Figure 3) and placed intermittent sutures to close the soft-tissue flaps. The patient was then seen by the prosthodontist for restorative treatment.
Restorative Treatment
A definitive impression was made of the Encode Healing Abutments. The abutments were removed and implant temporary cylinders were placed into the internal interface of the implants. The temporary cylinders were adjusted for interocclusal clearance and picked up inside the laboratory-processed provisional FPD. Ideal emergence profiles were developed and the provisional FPD was secured with abutment screws torqued to 20 Ncm. The provisional restoration had minimal occlusal contacts in centric occlusion. Lateral contacts were eliminated (Figure 4 and Figure 5).
During the osseointegration waiting period, the final Encode Abutments were fabricated. Three months after the implant placement, the provisional restoration was removed. Osseointegration was evaluated by applying 32 Ncm of reverse torque to the implants. The patient did not experience any discomfort with this procedure and macroscopic movement was not seen. The implants were considered to be osseointegrated. New implant temporary cylinders were placed into the implants and a verification index was made with autopolymerizing acrylic resin. Impression copings were placed into the internal interface of the implants and an implant level impression was made (Figure 6). A master cast was fabricated in conventional fashion. Wax patterns were developed to full contour. A facial silicone index was made of the previous wax patterns. The wax patterns were then cut back for porcelain application (Figure 7). The definitive crowns were fabricated on the Encode Abutments and the natural teeth (Figure 8).
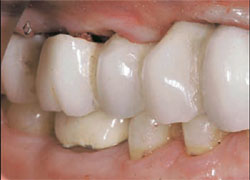
The patient returned for insertion of the abutments and implant crowns. The provisional FPD was removed. The Encode Abutments were seated and the abutment screws were torqued to 20 Ncm. The abutment placement was verified with radiographs before applying torque to the abutment screws. The access openings were blocked out. The crowns were tried in individually and together, and the occlusion was adjusted for both centric and eccentric movements. The crowns were cemented per conventional prosthodontic protocol (Figure 9 and Figure 10).
CLINICAL OVERVIEW
This clinical case demonstrated an accelerated treatment timeline by combining extraction, grafting, and placement of a fixed provisional FPD initially supported by teeth. After osseous healing, the hopeless tooth was extracted, and implants were placed and immediately restored with an implant-retained provisional FPD. Osseointegration and soft-tissue healing occurred at the same time. Total treatment time for this patient was approximately 8 months. With the more traditional, earlier protocols, this treatment would likely have taken 18 to 24 months because the treatment steps would have been done individually, in sequence. At least one more surgery would also have been required—uncovering the implants and placing the conventional healing abutments.
For additional case photographs and more information, please visit www.biomet3i.com.
DISCLAIMER
The authors have received grant/research support and honoraria from BIOMET 3i.
REFERENCES
1. Branemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1-132.
2. Adell R, Lekholm U, Rockler B, et al. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387-416.
3. Lazzara R, Porter S, Testori T. A prospective multicenter evaluation loading of OSSEOTITE Implants two months after placement: one-year results. J Esthet Dent. 1998;10(6):280-289.
4. Testori T, Del Fabbro M, Feldman S, et al. A multicenter prospective evaluation of 2-months loaded OSSEOTITE implants placed in the posterior jaws: 3-year follow up results. Clin Oral Implants Res. 2002;13(2):154-161.
5. Sullivan DY, Sherwood RL, Porter SS. Long-term performance of OSSEOTITE implants: A 6-year clinical follow-up. Compend Contin Educ Dent. 2001;22(4): 326-334.
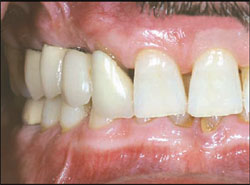 | 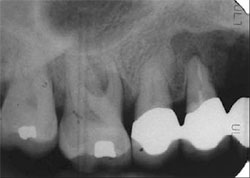 | ||
| Figure 1 Preoperative clinical photograph with patient in centric occlusion. | Figure 2 Preoperative periapical radiograph showing severe localized bone loss on the right maxillary molar and periapical radiolucency on the first premolar. | ||
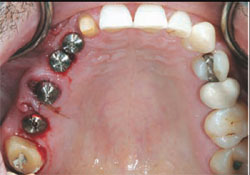 |  | ||
| Figure 3 Occlusal image of Encode Healing Abutments immediately after implant placement. | Figure 4 Buccal view of the immediate provisional FPD in centric occlusion. | ||
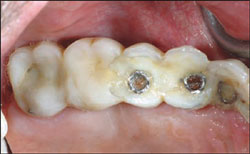 | 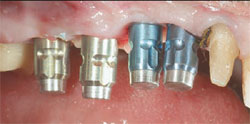 | ||
| Figure 5 Occlusal view of the immediate provisional FPD. | Figure 6 Implant transfer impression copings in place for making the definitive impression. | ||
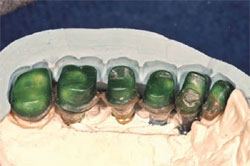 | 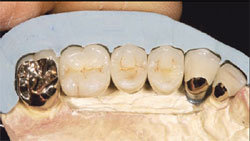 | ||
| Figure 7 Laboratory image of wax patterns for the implant FPD (silicone index in place). | Figure 8 Laboratory view of the definitive implant restorations with the silicone index in place. Note: The maxillary lateral incisor is a crown restoration restoring the natural tooth. | ||
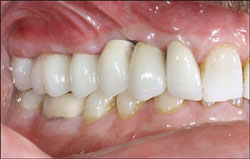 | 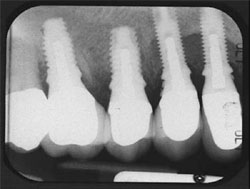 | ||
| Figure 9 Clinical photograph of the definitive restorations in place with the patient in centric occlusion. | Figure 10 Periapical radiograph of the definitive restorations on NanoTite PREVAIL Implants. Note: Smaller restorative components in place are consistent with the Platform Switching™ concept (BIOMET 3i). | ||
 | Robert Emery, DDS Board Certified, Oral and Maxillofacial Surgery, Private Practice Washington, DC | ||
 | Benjamin Watkins, DDS Fellow in Advanced Implant Prosthetic Education, Private Practice limited to Prosthodontics Washington, DC | ||
 | Steven A. Guttenberg, DDS, MD Board Certified, Oral and Maxillofacial Surgery, Private Practice Washington, DC | ||



