Correction of Latrogenic Gingival Recession in the Esthetic Zone
Paul S. Petrungaro, DDS, MS
In the contemporary reconstructive, cosmetic, or surgical dental practice, patients requesting cosmetic enhancement of their dentition have a much higher awareness of procedures that are available to them to correct/enhance their current dental health state. The replacement of old, ill-fitting restorations is commonplace in the modern practice, and the ability to manage any situation that may present itself has never been more demanding to the profession than now.
Esthetic enhancement of the existing dentition often requires alteration to the existing free gingival margin of the tooth/teeth to be treated. Crown-lengthening procedures are often used to balance gingival margins and to provide a symmetrical appearance of the gingival complex prior to veneers or full-coverage restorations being placed. This process is usually required when an excessive amount of keratinized gingival tissue is present, or when preexisting restorations are present and a biologic width invasion exists.
In the latter case, repositioning of the alveolar crest is usually required, along with apical repositioning of the free gingival margin of the gingival tissues present. To allow for adequate maturation of the new gingival complex, 2 to 3 months of healing are usually required before manipulation by the restorative clinician with routine cosmetic dental procedures.
Correction of the free gingival margin also may require the addition of soft tissue grafting for root coverage, free gingival grafting to increase the zone of keratinized tissue, sliding pedicle grafts for root coverage, and/or coronal repositioning of the free gingival margin. These procedures have been well documented in the dental literature.1-8 Sources of additional tissue are donor tissue from the palate, collagen membranes, or acellular dermal matrix grafts. The use of these products also has been well documented in the dental literature.9-13
A particular dilemma that presents itself is the lack of keratinized tissue facially in the presence of a preexisting restoration, where the facial margin of the preparation is too far apically positioned. This usually results in a lack of symmetry of the heights of contour of the existing gingival complex. The solution here is to lengthen the adjacent natural teeth to match the tooth/teeth in question, which may lead to excessive length of the teeth, or orthodontic extrusion to bring the margin of the preparation more coronally.
This article will outline a new approach to the correction of discrepancies in the facial heights of contour of adjacent teeth in the esthetic zone (due to a pre-existing restoration resulting in a biologic width invasion) using a combination of the aforementioned procedures, and demonstrating a new product to increase the thickness of gum tissue around natural teeth and/or dental implants.
Case Presentation
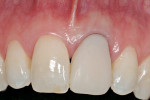
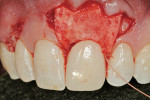
A 27-year-old non-smoking man presented for the esthetic enhancement of his left central incisor, which had a preexisting, full-coverage restoration that required replacement (Figure 1). The patient had begun orthodontic treatment in the mandibular arch to correct a minor occlusal imbalance and required coronal repositioning and soft tissue grafting to correct gingival recession at the facial of the right canine, in addition to correction of the free gingival margin at the facial of the left central incisor, which was asymmetrical to the free gingival margin of tooth No. 8. Additionally, the facial gingival tissues were of a thin biotype, which at the facial surface of the left central incisor allowed for the darkened root surface to be seen through the tissue.
The treatment plan was to accomplish an increased zone of keratinized tissue at tooth No. 6 and increase the thickness of the facial tissue around tooth No. 9, in addition to coronally repositioning the free gingival margin. Complicating this treatment plan was the fact that at the facial of tooth No. 9 a biologic width invasion was present, which was a result of the prepared margin for the pre-existing restoration being placed too close to the facial alveolar crest of bone. (The patient presented to the periodontist with a provisional restoration in place). Any correction to the area would require movement of the facial margin of the tooth in a coronal fashion to re-establish the appropriate distance from the facial height of bone to the margin of the restoration. Additionally, options were given to the patient for harvesting of the soft tissue, using connective tissue from the palate, or the use of an acellular dermal matrix graft, which would nullify harvesting tissue from the palate region.
The patient opted for the use of the acellular dermal matrix material for the procedure, along with platelet-rich plasma (PRP) enhancement to aid in the re-vascularization of the graft. The tissue selected for the augmentation procedure at teeth Nos. 6 and 9 was a dermal graft (Puros® Dermis Allograft Tissue Matrix [Zimmer Dental, Carlsbad, CA]) that was prepared by the Tutoplast® process (Tutogen Medical, Alcoa, FL).
Puros Dermis Allograft Tissue Matrix is recovered following the rigorous standards of both the Food and Drug Administration and the American Association of Tissue Banks (AATB) with either a scalpel or dermatome from the back of the thighs of the donor. The tissue is recovered within 24 hours of death using an aseptic process by a recovery team that meets the standards set by AATB. The tissue does not enter the Tutoplast process until it passes serologic tests (eg, HIV, hepatitis, human t-lymphotrophic virus, and syphilis). The Tutoplast process is a multi-step process that:14
- removes all antigenicity
- inactivates all kinds of pathogens
- preserves tissue structure and collagen
- preserves biomechanics
- guarantees sterility
- results in graft healing comparable to autografts
The process itself consists of:14
- donor selection
- osmotic treatment
- oxidative treatment
- alkaline treatment—different from bone
- solvent dehydration
- low-dose gamma irradiation
- full documentation
The Tutoplast process is fully validated for biomechanics, virus inactivation, prion inactivation, antigenicity, and healing. The Puros Dermis Tissue allograft has a strength-to-failure measurement of 5 pounds ± 0.8 and can be stored at room temperature, has a 5-year shelf life, and, because of the Tutoplast process, has no residual chemicals as it is packed and delivered to the clinician. Compared to the dermal matrix available on the market, which has a failure measurement of 4.5 pounds ± 1.1 and must be refrigerated, has only a 2-year shelf life, and is packed with a variety of residual antibiotics, the Puros Dermis Tissue offers a superior graft product to the clinician.
The enhancement with PRP has been well documented in the dental literature. PRP (autologous platelet gel) is developed from autologous blood with a cell separator.15-18 A 60-mL syringe is prefilled with 5 mL of a citrate-based anticoagulant. For each 60-mL syringe, approximately 45 mL to 55 mL of the patient’s blood is withdrawn from a venous puncture in the upper arm. The anticoagulated blood is dispensed into the blood chamber of the processing disposable. The blood must be drawn before the commencement of surgery, because surgery itself leads to platelet activation of the coagulation system.
A processing disposable is loaded into the centrifuge rotor cup of the Smart PReP™ Platelet Concentrate System (Harvest Technologies Corporation, Plymouth, MA). A counterbalance is then placed in the opposing rotor cup unless a second processing disposable is required. During processing, the blood is initially centrifuged at 3,650 rpm to separate the red blood cells from the plasma. The centrifuge then slows to approximately 60 rpm, allowing the plasma to automatically decant into the plasma chamber. The centrifuge then accelerates to 3,000 rpm to form a pellet of pure plasma concentrate in the bottom of the plasma chamber. The entire process of separating the whole blood into red blood cells, platelet-poor plasma (PPP), and platelet concentrate is completely automatic and completed in approximately 12 minutes.
The blood chamber of the process disposable contains red blood cells. The second chamber contains the platelet concentrate (a button-like precipitate) and PPP (supernatant). Approximately two thirds of the PPP are removed and can be saved for hemostatic applications. The platelet concentrate is then resuspended in the remaining PPP, thereby creating a very concentrated PRP solution.
The activator for the PRP and PPP is a mixture of 5,000 units of topical bovine thrombin and 5 mL of 10% calcium chloride. The activator is drawn into two 1-mL syringes and 10 mL of PRP is then drawn into one syringe and 10 mL of PPP is drawn into the other syringe. The two syringes are attached to a 20-guage dual cannula applicator tip where the contents are mixed as they are applied into/onto the bone graft, wound, connective tissue graft, or incisions.
The following growth factors are found in PRP: platelet-derived growth factor; transforming growth factor-beta; platelet-derived endothelial cell growth factor; platelet-derived angiogenesis factor; insulin-like growth factor; and vascular endothelial growth factor.15,16
After administration of an appropriate local anesthetic, correction of the gingival tissue at the facial of tooth No. 6 was accomplished in a fashion consistent with the soft tissue grafting technique that will be described below.
After removal of the provisional restoration, further examination of the prepared facial margin was made. Sounding measurements were obtained, and demonstrated a distance from the free gingival margin to the crest of bone at the mesial-facial point to be 2 mm, at the mid-facial point to be 2.5 mm, and at the distal-facial point to be 3 mm. Normally, crown lengthening could manage this problem; however, because of the esthetic and periodontal requirements of the esthetic dentist, and the esthetic desires of the patient, the existing margin of the tooth preparation needed to be moved in a more coronal fashion. The procedure required moving the facial margin of the tooth preparation coronally by root planing the existing margin off, and re-preparing a new facial margin 1 mm more coronally, followed by Puros Dermis grafting to increase the zone of tissue thickness, in addition to coronally repositioning the facial gingival margin.
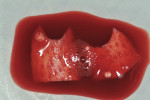
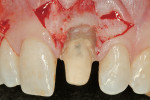
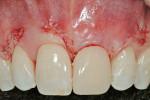
Before incision, the trimmed Puros Dermis was rehydrated in the PRP solution previously obtained (Figure 2). A broad-based, papillary spacing incision was made at the facial of tooth No. 9, followed by full-thickness, mucoperiosteal flap elevation. The facial root surface of the tooth exhibited notches and an uneven root surface. After extensive root planing of the facial root surface with hand root planing and rotary instrumentation to eliminate not only the notched root surface but also to eliminate the pre-existing facial tooth preparation margin, a new chamfer margin was prepared in the tooth 1 mm from the pre-existing margin (Figure 3). This was followed by application of a citric acid solution (pH 1) over the facial root surface for 1 minute to aid in sterilization of the root surface. Adjustment to the facial aspect of the preexisting provisional restoration by shortening the facial margin by 1 mm was accomplished, and the provisional restoration was cemented with temporary cement.
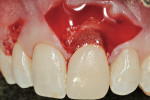
The outline of the pre-existing facial margin can be seen in Figure 3, approximately 0.75 mm from the facial aspect of the temporary restoration. Also note the symmetry between the facial aspect of the clinical crown on tooth No. 8, and provisional restoration of tooth No. 9. Preceding fixation of the Puros Dermis, application of PRP was performed (Figure 4). The PRP-enhanced Puros Dermis was then placed over the root surface (Figure 5) and secured with 4.0 Vicryl Rapide (Ethicon, Inc, Somerville, NJ) in a sling fashion around tooth No. 9. Additional application of PRP preceded a tension-free flap closure, totally covering the Puros Dermis with the pre-existing facial gingival tissues. Closure was accomplished with 4.0 Vicryl Rapide sutures using a horizontal mattress and continuous sling suturing method (Figure 6). Note the coronal repositioning of the facial gingival margin. Additional application of PRP over the surgical site served as a bioactive wound dressing.
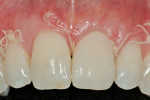
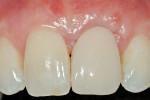
The 10-day postoperative view can be seen in Figure 7. Note the level of soft tissue healing at this early time frame. A 30-day postoperative view can be seen in Figure 8. A comparison with Figure 1 () demonstrates the new facial gingival margin, the thickness in biotype of tissue obtained, and the excellent color/tissue match obtained. A dermabrasion procedure will be necessary at the distal papillary tissue to remove the ledge of tissue before the restorative clinician proceeds with the new, full porcelain restoration at tooth No. 9, after an additional 30 days of healing and maturation.
Conclusion
The blending together of multiple procedures to achieve a desired result is often required with the contemporary reconstructive and surgical dental practice. The use of various allogenic materials in the surgical dental practice can allow the patient to undergo more advanced grafting procedures without the necessity of harvesting tissue from a donor site. The Puros Dermis Allograft Tissue Matrix has been clinically observed by the author to provide excellent soft tissue integration and tissue color matching when used as a soft tissue graft to increase the height and width of the zone of keratinized tissue. Additional controlled clinical studies and evaluations are necessary to determine the value of this material.
References
1. Grupe HE, Warren RF. Repair of gingival defects by a sliding flap operation. J Periodontol. 1956;27:290-295.
2. Napers JM. Free gingival grafts. Periodontics. 1966;4(5): 243-245.
3. Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application. Part I. Technique. Int J Periodontics Restorative Dent. 1982;2(1):65-70.
4. Miller PD Jr. A classification of marginal recession. Int J Periodontics Restorative Dent. 1985;5(2):8-13.
5. Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5(2):14-37.
6. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56(12):715-720.
7. Raetzke PB. Covering localized areas of root exposure employing the "envelope" technique. J Periodontol. 1985;56(7):397-402.
8. Harris RJ. The connective tissue and partial thickness double pedicle graft: a predictable method obtaining root coverage. J Periodontol. 1992;63(5):477-486.
9. Harris RJ. A comparative study of root coverage obtained with guided tissue regeneration utilizing a bioabsorbable membrane versus the connective tissue: with partial-thickness double pedicle graft. J Periodontol. 1997;68(8): 779-790.
10. Harris RJ. A comparison of 2 root coverage techniques: guided tissue regeneration with a bioabsorbable matrix style membrane versus a connective tissue graft combined with a coronally positioned pedicle graft without vertical incisions. Results of a series of consecutive cases. J Periodontol. 1998;69(12): 1426-1434.
11. Jepsen K, Heniz B, Halben JH, et al. Treatment of gingival recession with titanium reinforces barrier membranes versus connective tissue grafts. J Periodontol. 1998;69(3): 383-391.
12. Harris RJ. A comparison of root coverage obtained with a connective tissue graft versus acellular dermal matrix (abstract). J Periodontol. 1999;70:235.
13. Aichelmann-Reidy MB, Yukna RA, Mayer ET. An acellular dermal matrix used for root coverage (abstract). J Periodontol. 1999;70: 223.
14. Data on file at Tutogen Medical, Alcoa, FL.
15. Lynch SE, Genco, Marx RE (eds). Tissue Engineering: Applications in Maxillofacial Surgery and Periodontics. Hanover Park, IL: Quintessence Publishing Co; 1999:71-82.
16. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
17. Petrungaro PS. Immediate restoration of dental implants after tooth removal: a new technique to provide esthetic replacement of the natural tooth system. International Magazine of Oral Implantology. 2001;2(2): 6-16.
18. Petrungaro PS. Platelet-rich plasma for dental implants and soft-tissue grafting. Dental Implantology Update. 2001;11(6):41-47.
About the Author
Paul S. Petrungaro, DDS, MS
Director
The Institute for Advanced Dental Education, Inc
Lake Elmo, Minnesota
Private Practice
Chicago, Illinois and Lake Elmo, Minnesota