Characteristics of a New Glass Ionomer Material
Karl F. Leinfelder
Glass ionomers represent the newest class of agents to be used in the restorative process.1 Introduced nearly 30 years ago,2 they continue to play an ever-expanding role in the restoration of teeth. Although commonly used as an auxiliary in conjunction with composite resins,3 they also function successfully as a luting agent.4 Additionally, this class of restorative materials is used routinelyin the treatment of abfracted lesions,5 particularly in aging patients.
The clinical success of glass ionomers can be attributed to a number of factors. The first of these is the ability of the material to bond to tooth structure.6-8 It is generally recognized that ionic bonding is the principal mechanism of adhesion. The cement adheres to the apatite structure by hydrogen bonding. However, as the cement hardens, the hydrogen bonds are replaced by metal ions, thereby producing a metal ion bridge. The cement may also bond or adhere to the dentinal collagen through hydrogen and ionic bonding.9 Another factor is the coefficient of thermal expansion (CTE).10 Perhaps the most important characteristic of this particular factor is its matched CTE with natural tooth structure, particularly dentin. Consequently, the potential for microleakage and the development of caries at the preparation interface is diminished considerably. Also attributed to the matched CTE is the elimination of, or substantial reduction in, postoperative sensitivity. Many clinicians routinely place some form of a glass ionomer between the composite restoration and the floor of the preparation as their primary means for preventing sensitivity.11
Furthermore, the abundant release of fluoride ions from glass ionomers effectively kills the microorganisms associated with the caries process.12-14 Another basic advantage to glass ionomers is their ability to transfer fluoride ions into the adjacent tooth structure.15
Finally, glass ionomers can satisfactorily serve as a dentin substitute. Thisparticular attribute is highly desirable in association with current concepts of minimally invasive dentistry. Replacing defective dentin with glass ionomer rather than removing the entire undermined enamel can enhance treatment longevity in many instances. The most recent emphasis on the use of glass ionomer has been as aluting agent, and for numerous reasons. These include ease of use, fluoride release, and bonding potential to the restorative material and underlying tooth structure.
Glass Ionomers as a Luting Agent
Fuji Plus™ (GC America, Inc, Alsip, IL) is a new, resin-reinforced glass ionomer luting agent. The powder component is an aluminosilicate glass, while the liquid is an aqueous solution of polyacrylic acid, 2-hydroxyethyl methacrylate (2-HEMA), and tartaric acids. This resin-reinforced glass ionomer is designed for final cementation of various types of restorative materials, including metal, porcelain-fused-to-metal, and metal-free crowns, bridges, inlays, and onlays. It bonds chemically and mechanically to tooth structure and all types of core material. Its simple placement technique produces significantly higher bond strengths than conventional glass ionomer cements, while still maintaining the favorable characteristics of glass ionomers (ie, fluoride release, low CTE, soft- and hard-tissue biocompatibility).
The Fuji Plus glass ionomer system also is recommended for cementable reinforced all-ceramic crowns, including Procera® (Nobel Biocare™ USA, Inc, Yorba Linda, CA), and InCeram (Vident™, US distributor of Vita Zahnfabrik, Brea, CA). It is of course recommended for cementable composite resin restorations, such as Gradia® (GC America). Fuji Plus also can be used for the cementation of orthodontic bands.
Formerly known as Fuji Duet, this modified formulation is not only recommended for broader application, but is less complex to use. For example, a preluting conditioner is no longer required; it is an optional step if a higher bond strength to enamel is desired. The use of the conditioner elevates the bond strength from 9.5 MPa to 17 MPa. Furthermore, the newer formulation does not require sealing the exposed margins with a light-activated bonding resin.
The Fuji Plus formulation demonstrates a working time of 2 minutes or 2.5 minutes, depending on the mixing technique. The shortest working time of 2 minutes is obtained routinely through the use of the capsulated system. Setting time for both activation methods is 5 minutes.
In addition to the properties already described, Fuji Plus is relatively easy to use. As a result of its flowable characteristics and film thickness (10 µm), full seating of the restoration or prosthesis onto the preparation is better assured. The working time of the glass ionomer facilitates cementation of long-span bridges, multiple-abutment bridges, and multiple-unit restorations. Furthermore, the use of the encapsulated material in conjunction with a mechanical mixing device ensures optimal mechanical properties, decreases chair time, and eliminates clean-up time.
Direction for Cementation
Application of Fuji Plus conditioner to the surface of the preparation is optional. It does prepare the bonding surface, dramatically increases the bond strength, and reduces the chance for pulpal sensitivity. A 20-second application of Fuji Plus conditioner removes the smear layer with a mild citric acid etchant and seals the dentinal tubules with its ferric chloride component. The same conditioner also can be used to treat the composite resin core.
Apply the mixed Fuji Plus to the inside of the restoration. This should be accomplished by applying a thin layer of the luting agent to the internal surface of the restoration with a microbrush. The working time for hand mixed is 2.5 minutes; capsules is 2 minutes.
Place the restoration under moderate finger pressure or by means of an appropriate subsonic and quickly remove excess cement when rubbery (approximately 30 seconds). As is the case with nearly all luting systems, the area should be kept dry. Refrigeration will extend the working time.
Conclusion
In addition to enhanced luting capabilities, the uses of this type of formulation have been extended; and its characteristics include the following:
- Very low film thickness (10 µm)
- Smooth, creamy consistency
- Crown seating potential is increased
- Conditioner is optional
- Enhanced dentin and enamel bond strengths
- New capsule design is simpler to use
- Easier to mix and insert
- Extended working time
- Ideal setting time
- High fluoride release
- Matched CTE with tooth structure
- Clinically insoluble when set
- Ionic bonding to tooth structure and metals
- Maintains marginal seal
- Excellent biocompatibility
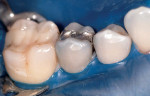
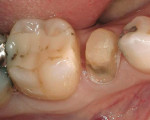
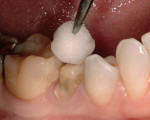
The development of the Fuji Plus glass ionomer system represents an advancement in resin-reinforced glass ionomer materials. (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6)
References
1. Katsuyama S, Ishikawa T, Fujii B. Glass ionomer dental cement. 1993; Ishiyaku Euro America, Inc, St. Louis, Mo.
2. Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972;132(4):133-135.
3. McLean JW, Powis DR, Prosser HJ, et al. The use of glass-ionomer cements in bonding composite resins to dentine. Br Dent J. 1985;158(11):410-414.
4. Horn HR. The current status of dental luting cements. NY State Dent J. 1983;49(8)549-551.
5. Brandau HE, Ziemiecki TL, Charbeneau GT. Restoration of cervical contours on nonprepared teeth using glass ionomer cement: a 4 1/2-year report. J Am Dent Assoc. 1984; 108(5):782-783.
6. Hotz P, McLean JW, Sced I, et al. The bonding of glass ionomer cements to metal and tooth substrates. Br Dent J. 1977; 142(2):41-47.
7. Coury TL, Willer RD, Miranda FJ, et al. Adhesiveness of glass-ionomer cement to enamel and dentin: a laboratory study. Oper Dent. 1982;7(1):2-6.
8. Vougiouklakis G, Smith DC. Bonding of restorative materials to teeth. J Dent Res. 1978;57:340.
9. Phillips RW. In: Skinner’s Science of Dental Materials. 8th ed. 1982; WB Saunders, Philadelphia, Pa; 472.
10. Bullard H, Leinfelder KF, Russell CW. Effect of coefficient of thermal expansion on microleakage. J Am Dent Assoc. 1988; 116:871-874.
11. Leinfelder KF. Glass ionomers: current clinical developments. J Am Dent Assoc. 1993; 124:62-64.
12. Forsten L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977; 85(6):503-504.
13. Onose H. Study on the antibacterial effects of glass ionomer cement. Biocompat Dent Mater. 1977;20:130.
14. Onose H. Study on the antibacterial effects of the glass ionomer cement. J Conserv Dent. 1977;20(2):406-409.
15. Koulourides T, Keller SE, Manson-Hing L, et al. Enhancement of fluoride effectiveness by experimental cariogenic priming of human enamel. Caries Res. 1980;14(1):32-39.
About the Author
Karl F. Leinfelder, DDS, MS
Adjunct Professor, Biomaterials Clinical Research
University of North Carolina
Chapel Hill, North Carolina
Professor Emeritus
University of Alabama School of Dentistry
Birmingham, Alabama