You must be signed in to read the rest of this article.
Registration on AEGIS Dental Network is free. Sign up today!
Forgot your password? Click Here!
Sulcular Modified Internal Labial Enhancement (SMILE) Technique for Gingival Augmentation
David Anson, DDS; and Robert A. Horowitz, DDS
The goals of soft-tissue augmentation in periodontics, also known as periodontal plastic surgery, are wide ranging. They include coverage of the root(s) up to the cementoenamel junction (CEJ) for esthetics, reduced temperature sensitivity (if necessary), protection of exposed cementum from further wear, good color match to adjacent tissue, and proper contour. Also, other desirable objectives, if possible, are to develop sufficient attached keratinized tissue, eliminate frenum pulls, obtain probing depths equal to or less than 3 mm, have no bleeding on probing, and minimize patient discomfort.1
There are limitations to the amount of root coverage that may be obtained, because the direct facial soft tissue will always be apical to the height of the papillae (scalloped morphology), and the papillary position cannot be predictably moved coronally if it is apical to the CEJ. Therefore, with interproximal recession full root coverage is not possible. Additionally, the most predictable results are obtained when at least 3 mm exists between adjacent roots so that the papillae have a mesial-distal width of at least 3 mm.2
Like other techniques in dentistry, procedures used for soft-tissue augmentation of recessed roots have evolved over many years. The emphasis today is on minimally invasive procedures, with practitioners striving for greater predictability with decreased local morbidity. The evolution in root coverage started with the use of the free gingival graft when a laterally positioned or coronally positioned graft was not applicable.3 In a recent observational long-term study, treated sites that increased the amount of keratinized tissue with free gingival grafting resulted in less recession over the 25-year study period than untreated sites.4 Significant drawbacks of this technique, however, are the requirement of a second surgical site to obtain the donor tissue, which leads to greater morbidity, and a lack of blending with adjacent tissue with regard to color and contour. The connective tissue graft by Langer and Langer later became the preferred technique in periodontics due to less morbidity to the palate, better blood supply at the recipient site, and good color match and contour.5 With these techniques, however, a limited supply of autogenous tissue from the palate restricts the number of teeth that may be treated per surgery.
Newer techniques have evolved that have eliminated the need for autogenous tissue from the palate, allowing many more teeth to be treated in a single surgery.6,7 These techniques generally use tissue allografts or xenografts, along with various tunneling techniques. One newer method by Chao, the pinhole surgical technique (PST), represents an evolution of the tunneling approach with the novel aspect of not utilizing suturing.8 A systematic review from the American Academy of Periodontology's Regeneration Workshop assessed a number of techniques, including subepithelial connective tissue-based procedures and coronally advanced flap plus acellular dermal matrix grafts, enamel matrix derivative, and collagen matrix procedures.9 Connective tissue graft-based procedures yielded superior percentages of mean and complete root coverage, as well as significant increase of keratinized tissue. Tunneling techniques, however, were not reviewed.9
With the aforementioned PST technique, Chao observed that there was less surgical time and less patient morbidity than with connective tissue grafting when comparing his results to a study of connective tissue grafting by Wessel and Tatakis.10 However, in another study comparing connective tissue grafts to a tunnel technique, the opposite was found.11 These conflicting results may be due to varying approaches used in both the tunnel and connective tissue techniques. The gingival sulcular modified internal labial enhancement (SMILE) technique, which the present authors are describing herein, is yet a different approach to the tunneling technique, with the addition of platelet-rich fibrin (PRF). The present authors have extensive experience with connective tissue grafting and unscientifically have observed outcomes similar to the Chao study.8 Regarding that study as related to the mean percentage of defect coverage, the PST technique yielded similar results to a study by Chambrone and Chambrone that evaluated results obtained with a connective tissue graft placed under a coronally advanced flap for the treatment of multiple gingival recessions defects.12 Both of those studies also reported a higher mean percentage of root coverage obtained when comparing results of the maxillary arch versus the mandibular arch.8,12 Root surface treatments (eg, enamel matrix derivative, lasers, tetracycline, etc) do not seem to have an additive effect on the percent of root coverage obtained with connective tissue grafting,13 but the effect on procedures not utilizing connective tissue needs to be studied. Also, root surface treatments need to be studied regarding effects on patient morbidity and histologic outcomes.
The use of PRF in soft-tissue healing has been studied with positive results being shown regarding healing time and morbidity.14,15 Its effects on the long-term results regarding root coverage, however, have not shown to be significant.16,17 This article describes a procedure, the aforementioned SMILE technique, that uses a tunneling technique with a xenograft and PRF and requires significantly fewer instruments than other comparable techniques.7,8
Technique Protocol
Like any root coverage procedure, thorough root surface debridement is the critical initial step in the SMILE technique. This can be accomplished with hand instrumentation, a high-speed handpiece, and ultrasonic instrumentation. Root surface filings should collect on the face of the instrument during hand instrumentation. If a composite restoration has been previously placed, it can be removed with high-speed burs and hand instrumentation to obtain a flat, clean root surface. Also, there should be a smooth transition from the root surface to the facial enamel. Root surface debridement is best accomplished before the tunnel is developed because the field is drier, the total tissue release time will be reduced, and there will be less contamination under the soft tissue. If, however, the exposed root surface contains a very large concavity or the recession dimension is extremely thin mesio-distally, it may be necessary to develop the tunnel to completely prepare the root surface.
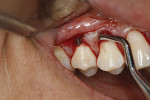
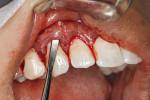
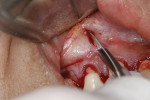
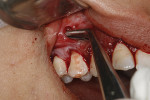
After thorough root debridement has been accomplished, the facial soft tissue is released. This procedure starts with a sulcular approach initially using a sulcular papillary elevator developed by the author (Dr. Anson) (H&H Company, hhcompany.store) (Figure 1). The sharp instrument is inserted underneath the interproximal tissue, and the facial papillary tissue is carefully raised using a gentle rocking motion, reaching full thickness and slowly advancing deeper, approaching the papillae mesially and distally until the instrument has progressed fully underneath the tissue and re-emerges out the other end (Figure 1). A sharp Fedi chisel is then used facially and interproximally to gently release the rest of the sulcular tissue, with extreme care taken to avoid piercing the tissue or tearing the papillae (Figure 2). Full-thickness elevation of the gingiva is performed, but when the chisel reaches the mucosa, a split-thickness release will be utilized to gently stretch the tissue facially. Once the gingiva is released, a small incision, approximately 5 mm to 6 mm long, is made deep in the vestibule in the canine area (Figure 3). The incision should not be in the direct facial of the tooth; as with the coronal positioning the incision could be directly on the root surface. When treating the anterior areas, often an additional incision is made in the midline, especially when there is a frenum pull.
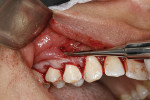
At this point, the Fedi chisel is used to release the facial tissue further in a split-thickness fashion. The instrument is inserted through the apical incision and moved back and forth, horizontally and vertically, to release and elevate the tissue (Figure 4). In areas of thin tissue and/or bony ridges where the clinician cannot fully release the marginal tissue with the sulcular approach, the Fedi chisel can be maneuvered through the apical incision to separate the attached tissue. The tissue should be able to be moved coronally to the CEJ and, if possible, beyond, with care again taken to not tear the papillae (Figure 4). The Fedi chisel is also used interproximally to raise the papillae as much as possible without tearing it. On the mandibular arch care must be exercised around the mental nerve area to prevent damage to the nerve. Because a split-thickness approach is used in the mucosa, this is usually not a problem, but the clinician needs to be cognizant of the nerve location.
The next step is to place the xenograft and PRF. A collagen xenograft made from porcine peritoneum (Straumann® Membrane Flex™, Straumann, straumann.com) that, according to the manufacturer, resorbs in 12 to 16 weeks is cut into strips with the approximate dimension of 4 mm x 10 mm. Usually a 30 mm x 40 mm piece of membrane is required per sextant. The collagen strips are then soaked in the platelet-poor solution obtained in the PRF procedure and placed through the apical incisions. Positioning the collagen strips starts with placement under the papillae and partially positioning them with cotton pliers (Figure 5). The PRF membranes are processed as described by Dohan and Choukroun et al.14 After the initial fibrin clots are removed from the red tubes provided in the PRF kit, they are compressed in the metal perforated "box" also included with the PRF kit. The liquid expressed into the bottom of the box is used to hydrate the collagen membranes. The collagen membranes should not be placed through the sulcular area, as this can potentially tear the papillae. The Fedi chisel may then be used to position the strips more posteriorly and coronally to support the papillae with gentle pressure. Additional strips are then used facially to passively position the tissue in a coronal position. Some of the collagen strips may be left exposed through the sulcus. The PRF membranes are then cut into pieces (Figure 6) and inserted into the surgical site. Suturing is not recommended because the graft materials may inadvertently be pushed apically. Upon completion of the surgery, wet gauze is placed in the vestibule for about 3 to 5 minutes to provide hemostasis and flap stability (Figure 7).
Postoperative instructions include avoidance of oral hygiene in the surgical area for 3 to 4 days, and then use of a dry, ultra-soft toothbrush (PHB Toothbrushes, phbtoothbrushes.com) in an apical to coronal direction, twice per day, along with an antiseptic mouthwash. The patient is also directed to avoid flossing the treated area for 6 weeks. Either chlorhexidine or an essential oils mouthrinse (eg, Listerine) can be used, but they may have a deleterious effect on fibroblasts (dose dependent in vitro), and chlorhexidine may cause staining when used long-term.18 Typically, just nonsteroidal anti-inflammatory drug (NSAID) medication is necessary postoperatively, and antibiotics are given (eg, 1 gram of amoxicillin orally presurgically, then one capsule three times per day for 7 days; if the patient is allergic to amoxicillin, clindimycin 150 mg may be prescribed, two capsules presurgically then one capsule four times per day or two capsules twice per day for 7 days, or azithromycin as directed).
Case Reports
Case 1
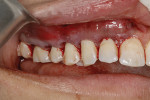
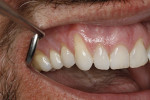
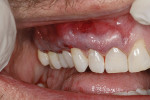
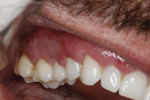
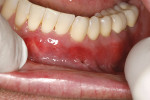
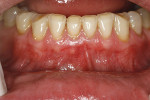
A 48-year-old male patient presented with generalized temperature sensitivity, progressing recession, cervical abrasion and abfractions, and an acknowledgment of being very aggressive with his oral hygiene (Figure 8). His health history was non-contributory. The SMILE tunneling procedure was performed as previously described, including the use of PRF. The treated area ranged from teeth Nos. 2 through 8, extending two papillae anterior to the area with the recession.
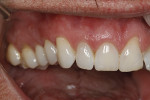
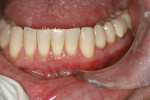
The patient was given amoxicillin 500 mg, #22, two capsules immediately, then one capsule three times per day for 1 week postoperatively. Figure 9 through Figure 11 show the results immediately post-surgery, 1 week postoperative, and 5 months postoperative, respectively.
Case 2
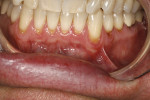
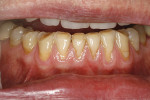
The patient was a 53-year-old man who presented with several mandibular teeth with cervical abrasion with a frenum pull facial at tooth No. 22 (Figure 12). He clenched, bruxed, and reported being very aggressive with his oral hygiene. He also stated that his wife had a bad experience with a gingival autograft performed by a different dentist.
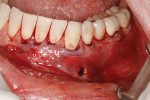
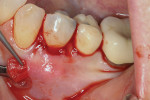
The procedure was performed on his left mandibular quadrant with PRF as described earlier (Figure 13), and healing was uneventful. The surgery extended from the distal of tooth No. 18 to the mesial of tooth No. 25; the right mandibular quadrant was treatment planned for later treatment. Figure 14 shows the 1-week postoperative result, and Figure 15 shows the 5-month postoperative view with full root coverage and elimination of the frenum pull on the marginal tissue.
Case 3
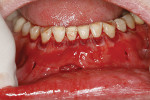
A 59-year-old male patient presented with a complaint of mandibular anterior recession that he believed was progressing (Figure 16). In addition to recession on multiple mandibular teeth, there was associated mucogingival involvement and cervical composites on the facials of teeth Nos. 22, 23, and 25. He was a bruxer and reported wearing a nightguard regularly.
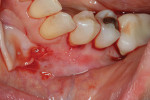
The composites on Nos. 22, 23, and 25 were removed with a high-speed bur, and all of the mandibular anterior roots were thoroughly root planed. No root conditioning was performed. Three incisions were made apically, one each at approximately the distal line angles of the canines and one in the area of teeth Nos. 25-26, and the procedure was completed as previously described. No sutures were used (Figure 17). Damp gauze was then placed facially for several minutes to obtain hemostasis. At 5 months postoperative an increase in the amount of attached keratinized gingiva was evident (Figure 18).
Discussion
The harvesting of autogenous tissue from a patient's palate has limitations, including discomfort and numbness, whether real or perceived, and anatomic insufficiency. For these reasons, alternative allogeneic and xenogenic tissue solutions are often sought.19-23 In an effort to increase biologic activity to improve root coverage, some researchers have hydrated these graft materials in platelet-rich plasma (PRP), growth enhancers that are taken from the patient's own blood. Shepherd and coworkers hydrated acellular dermal matrix in PRP, which was inserted through a tunnel procedure.24 They compared treated patients whose dermis was hydrated in saline or PRP. The results showed 70% root coverage in the saline group and 90% in the PRP group. Although both groups showed clinical improvement, the incorporation of PRP into the treatment regime did not result in statistical significance.
In the present study, in which a novel, minimally invasive approach for root coverage was utilized, allogeneic tissue was hydrated in liquid exudate from the formation of PRF barriers.25-27 Small amounts of platelet-derived growth factor-BB (PDGF-BB), insulin-like growth factor-1 (IGF-1), and transforming growth factor beta 1 (TGF-β1) have been found in the liquid hydrating the graft.25 However, with the present technique, after the graft is inserted into the tunnel, additional barriers of PRF are also placed into the surgical site. These barriers are formed differently than a PRP clot. When PRP is made, the fast polymerization caused by the extrinsic thrombin leads to a quick release of the growth factors trapped in the membrane. Because PRF develops through a slow cicatrization process, these materials are released over a significantly longer timeframe.28,29 The combination of surgical approach, barrier, and PRF all may contribute to the successful outcome of the cases presented herein.
Other researchers have incorporated PRF into surgical root coverage procedures with more traditional surgical approaches. Horowitz used PRF either alone or to hydrate and then insert with other collagen barriers.30 Excellent results were achieved at 2 to 6 months and maintained for 5 years. Öncü showed that PRF membranes compared favorably relative to subepithelial connective tissue (SECT) grafting under coronally advanced flaps.17 He showed that the SECT treated sites had a higher percentage of root coverage, and there was no statistically significant difference in full root coverage between the SECT and PRF treated sites. Additionally, there was significantly less patient discomfort in the PRF treated sites in the first postoperative week. Moraschini et al conducted a meta-analysis on PRF in the treatment of gingival recession and did not see an improvement in the amount of keratinized tissue width, root coverage, or attachment level when PRF was used.31
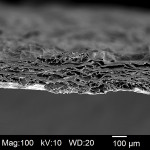
The xenograft barrier used in this case study is a resorbable, highly conformable, biomechanically strong collagen membrane manufactured from purified porcine peritoneum. The peritoneal tissue was purified through a proprietary series of treatments to remove non-collagenous components. After purification, the membrane was gently cross-linked to stabilize the matrix for predictable performance and resorption time. Upon completion of the stabilization step, removal of the cross-linking agent ensured a highly biocompatible membrane. The final product was then cut into various sizes and sterilized by gamma irradiation. The membrane itself is significantly porous, as can be seen in a high-resolution, cross-section scanning electron microscope (SEM) image (Figure 19). This porosity enables absorption of the liquid squeezed from the PRF when it is removed from the tubes and compressed in the perforated metal box. The substantial surface area also enables adsorption of proteins and growth factors onto the collagen itself.
PRF was used in this case report in two different ways. Collagen membranes were hydrated in the liquid exuded from compressed PRF barriers, or "clots," after their preparation. This can enable binding and long-term release of growth factors. To ensure that the collagen membrane stays in its precise location when inserted through the vertical incision, it is held in place with a small instrument (Micro Pliers Straight, Paradise Dental Technologies, pdtdental.com) (Figure 20), which allows the insertion of PRF barriers in either small chunks or full-length membranes (Figure 21) directly underneath the collagen membrane. Due to their method of fabrication, the PRF membranes will release growth factors for up to 4 weeks,29 which may have contributed to the excellent healing showed in this study.
Future study of this method should examine several options. Treatment of the root surface with ethylenediaminetetraacetic acid (EDTA) may improve the surface of the dentin and its ability to obtain true periodontal regeneration. Similarly, the addition of enamel matrix derivative has been shown to predictably enable both root coverage and the formation of true periodontal regeneration.32-34 Long-term studies will determine if these results hold up over many months and years. Lastly, digital scanning technology may enable researchers to obtain more accurate analysis of both vertical root coverage and determination of the thickness of tissue resulting from this combined approach. Improving a patient's biotype may have applications in pre-prosthetic and implant therapies for both cosmetic enhancement and long-term preservation of gingival margin levels.
Conclusion
The technique presented is a modification of current soft-tissue root coverage methods. It typically requires only a sulcular papillary elevator and several other commonly used instruments. The technique uses a sulcular approach as well as an apical one, and the material is a combination of collagen membrane strips and PRF. No sutures are used in the technique, and postoperative discomfort is comparable to other current methods of soft-tissue root coverage with a tunneling technique. A secondary surgical site is not needed, which may enhance patient acceptance. Quadrants and/or arches can be treated at one surgical appointment, because the technique is not limited by the amount of attainable autogenous tissue, and results are predictable. The addition of PRF to the technique may not change long-term results regarding the amount of root coverage, but it may facilitate faster healing and less morbidity postoperatively. A further area of study would be to compare this technique with other similar techniques.
About the Authors
David Anson, DDS
Private Practice, Beverly Hills, California
Robert A. Horowitz, DDS
Adjunct Clinical Assistant Professor, Periodontology and Implant Dentistry,
New York University College of Dentistry, New York, New York; Private Practice, Scarsdale, New York
References
1. Harris RJ. The connective tissue and partial thickness double pedicle graft: a predictable method of obtaining root coverage. J Periodontol. 1992;63(5):477-486.
2. Pini-Prato G, Magnani C, Zaheer F, et al. Influence of inter-dental tissues and root surface condition on complete root coverage following treatment of gingival recessions: a 1-year retrospective study. J Clin Periodontol. 2015;42(6):567-574.
3. Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in deep-wide recession. Int J Periodontics Restorative Dent. 1985;5(2):14-37.
4. Agudio G, Chambrone L, Pini Prato G. Biologic remodeling of periodontal dimensions of areas treated with gingival augmentation procedure: a 25-year follow-up observation. J Periodontol. 2017;88(7):634-642.
5. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56(12):715-720.
6. Allen EP. Subpapillary continuous sling suturing method for soft tissue grafting with the tunneling technique. Int J Periodontics Restorative Dent. 2010;30(5):479-485.
7. Zadeh HH. Minimally invasive treatment of maxillary anterior gingival recession defects by vestibular incision subperiosteal tunnel access and platelet-derived growth factor BB. Int J Periodontics Restorative Dent. 2011;31(6):653-660.
8. Chao JC. A novel approach to root coverage: the pinhole surgical technique. Int J Periodontics Restorative Dent. 2012;(32)5:521-531.
9. Chambrone L, Tatakis DN. Periodontal soft tissue root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86(2 suppl):S8-S51.
10. Wessel JR, Tatakis DN. Patient outcomes following subepithelial connective tissue graft and free gingival graft procedures. J Periodontol. 2008;79(3):425-430.
11. Gobbato L, Nart J, Bressan E, et al. Patient morbidity and root coverage outcomes after the application of a subepithelial connective tissue graft in combination with a coronally advanced flap or via a tunneling technique: a randomized controlled clinical trial. Clin Oral Investig. 2016;20(8):2191-2202.
12. Chambrone LA, Chambrone L. Subepithelial connective tissue grafts in the treatment of multiple recession-type defects. J Periodontol. 2006;77(5):909-916.
13. Karam PS, Sant'Ana AC, de Rezende ML, et al. Root surface modifiers and subepithelial connective tissue graft for treatment of gingival recessions: a systematic review. J Periodontal Res. 2016;51(2):175-185.
14. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-e44.
15. Ozcan M, Ucak O, Alkaya B, et al. Effects of platelet-rich fibrin on palatal wound healing after free gingival graft harvesting: a comparative randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2017;37(5):e270-e278.
16. Eren G, Atilla G. Platelet-rich fibrin in the treatment of localized gingival recessions: a split-mouth randomized clinical trial. Clin Oral Investig. 2014;18(8):1941-1948.
17. Öncü E. The use of platelet-rich fibrin versus subepithelial connective tissue graft in treatment of multiple gingival recessions: a randomized clinical trial. Int J Periodontics Restorative Dent. 2017;37(2):265-271.
18. Wyganowska-Swiatkowska M, Urbaniak P, Szkaradkiewicz A, et al. Effects of chlorhexidine, essential oils and herbal medicines (salvia, chamomile, calendula) on human fibroblast in vitro. Cent Eur J Immunol. 2016;41(2):125-131.
19. McGuire MK, Scheyer ET. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J Periodontol. 2010;81(8):1108-1117.
20. Sanz M, Lorenzo R, Aranda JJ, et al. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol. 2009;36(10):868-876.
21. Herford AS, Akin L, Cicciu M, et al. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J Oral Maxillofac Surg. 2010;68(7):1463-1470.
22. Allen EP. AlloDerm: an effective alternative to palatal donor tissue for treatment of gingival recession. Dent Today. 2006;25(1):48-52.
23. Clozza E, Suzuki T, Kye W, et al. Mucogingival volumetric changes after root coverage with acellular dermal matrix: a case report. Clin Adv Periodontics. 2014;4(4):256-262.
24. Shepherd N, Greenwell H, Hill M, et al. Root coverage using acellular dermal matrix and comparing a coronally positioned tunnel with and without platelet-rich plasma: a pilot study in humans. J Periodontol. 2009;80(3):397-404.
25. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-e50.
26. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-e55.
27. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56-e60.
28. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63-69.
29. Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012;13(7):1145-1152.
30. Horowitz RA. Optimizing root coverage with L-PRF. Inside Dentistry. 2011;7(10):66-76.
31. Moraschini V, Barboza Edos S. Use of platelet-rich fibrin membrane in the treatment of gingival recession: a systematic review and meta-analysis. J Periodontol. 2016;87(3):281-290.
32. Rasperini G, Silvestri M, Schenk RK, Nevins ML. Clinical and histologic evaluation of human gingival recession treated with a subepithelial connective tissue graft and enamel matrix derivative (Emdogain): a case report. Int J Periodontics Restorative Dent. 2000;20(3):269-275.
33. McGuire MK, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: Comparison of clinical parameters. J Periodontol. 2003;74(8):1110-1125.
34. McGuire MK, Cochran DL. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histological evaluation. J Periodontol. 2003;74(8):1126-1135.