You must be signed in to read the rest of this article.
Registration on AEGIS Dental Network is free. Sign up today!
Forgot your password? Click Here!
Framing the Restorative Result: How Tissue Augmentation and Preservation Maximizes Implant Therapy Outcomes
Barry P. Levin, DMD
Abstract
The role of hard- and soft-tissue augmentation as it pertains to dental implant therapy is often underestimated. If this restorative aspect is neglected during the natural healing process and subsequent remodeling following extraction(s), the long-term outcome of treatment can be catastrophic. Three of the most common opportunities for surgeons to enhance outcomes with regenerative therapy are: reconstruction of edentulous segments of the alveolus, management of extraction sockets, and immediate implant placements. Each has its own unique nuances, which can be quite confounding without an appreciation for the potential biomaterials and techniques currently available for use in these situations. This article will focus on novel methods aimed toward the reconstruction and maintenance of hard- and soft-tissue volumes needed to support functional, hygienic, and esthetic implant restorations.
Partially edentulous patients seeking fixed restoration of missing teeth often present deficient of necessary hard and soft tissue required to fulfill their desire for implant-supported prostheses. Three-dimensional ridge reconstruction can correct this insufficiency and facilitate implant-based restorative dentistry. The morbidity associated with harvesting of autogenous block grafts may result in patients foregoing implant treatment. The alternative of a semi-rigid mesh, often made of titanium, can serve as a scaffold for bone reconstruction. Not new to dentistry,1 this concept has become commonly used for reconstruction of resorbed alveolar ridges associated with implant therapy. Often, to minimize the use of secondary sites for bone acquisition, exogenous bone graft materials and recombinant growth factors are placed between the alveolar ridge and titanium mesh.2 What cannot be avoided with titanium mesh, however, is the need for invasive surgery to remove the mesh at the time of implant placement.
One strategy aimed at eliminating this drawback is the use of a bioresorbable mesh composed of a poly lactic-co-glycolic acid(PLGA) polymer (eg, RapidSorb®, DePuy Synthes, depuysynthes.com). The author has published several articles demonstrating this material as an effective alternative to titanium mesh.3-5 For reconstruction of severe vertical and horizontal alveolar bone deficiencies, space maintenance between the bone and mucosa is critical. Placement of an osteoconductive and osteoinductive graft underneath the PLGA mesh provides a scaffold to stimulate bone regeneration that the rigid mesh protects from collapse of the soft tissues. After approximately 6 months of healing, implants can be placed in prosthetically oriented positions.6 Additionally, because flap reflection to remove the mesh is unnecessary due to its resorbability, oftentimes a flapless, computer-guided implant placement can be performed.
The management of extraction sites is critical when implant-supported prostheses are planned. In many situations, immediate implant placement can be performed, as will be discussed later in this article. In circumstances where implants cannot be placed at the time of extractions, such as sites with active infections, wide areas of bone destruction, or proximity to vital structures like maxillary sinuses or inferior alveolar nerves, regenerative therapy can be employed to prevent the significant bone loss that occurs following extractions.
Often, the extraction socket is filled with inflammatory soft tissue that is associated with infections caused by root fractures, periodontitis, or endodontic lesions. Meticulous debridement must be performed to prevent contamination of bone graft materials. After mechanical socket cleansing, local application of antimicrobial agents, such as doxycyline or chlorhexidine, can be performed to further render the socket free of bacterial contamination. Then, a particulate bone graft material can be placed into the site. Graft material options range from autogenous, allogeneic, xenogeneic, or alloplastic. The author prefers using an allograft bone for these sites. This material is osteoconductive and capable of supporting bone apposition and replacement by native bone. Iasella et al demonstrated mineralized allograft capable of preservation of ridge dimensions when combined with a collagen barrier compared with extractions alone.7
Grafting extraction sockets with a particulate bone graft is often combined with placement of membranes capable of soft-tissue exclusion. Various membranes are commercially available. These materials are often composed of animal- or human-derived collagen. The author prefers a collagen membrane of porcine origin that is cross-linked with ribose sugar. This material has demonstrated not only barrier membrane properties but the ability to serve as a nidus for ossification.8 The resorbable nature of this collagen membrane presents the potential opportunity for minimally invasive, computer-guided implant placement. The following case demonstrates this clinical technique.
Minimally Invasive, Computer-Guided Implant Placement
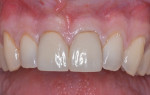
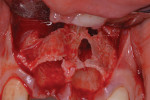
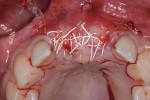
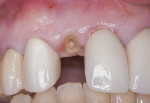
The patient was a 23-year-old man with a history of trauma and endodontic therapy associated with teeth Nos. 8 and 9 (Figure 1). Due to the acute and destructive nature of the infection present, immediate implant placement was contraindicated. After administration of local anesthesia, a facial flap was reflected, preserving the papilla associated with the mesial surfaces of the adjacent lateral incisors. The two involved teeth were extracted and the sockets were debrided with manual and ultrasonic curettes (Figure 2). Gauze squares were saturated with a slurry of doxycycline and sterile saline and placed into the sockets for approximately 3 minutes.
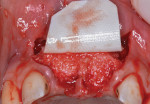
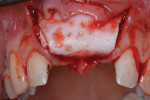
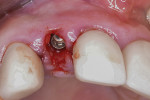
Next, copious irrigation with sterile saline preceded augmentation with a mineralized freeze-dried bone allograft (FDBA) (Symbios®, Dentsply Sirona, dentsplyimplants.com). A ribose cross-linked collagen membrane (Ossix® Plus, Datum Dental, ossixdental.com) was placed over the facial, crestal, and palatal ridge (Figure 3). Then, a dermal allograft (Symbios PerioDerm, Dentsply Sirona) was adapted over the collagen membrane (Figure 4). This was intended to increase the thickness of the soft tissues and act as a secondary membrane. The flaps were replaced without intentional coronal positioning of the facial flap, which would have resulted in relocation of the mucogingival junction toward the crest of the ridge (Figure 5).
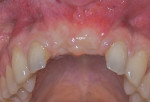
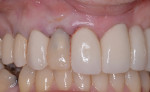
A healing period of approximately 6 months preceded implant placement (Figure 6). Because hard and soft tissues were sufficiently reconstructed and the mucogingival junction was not coronally advanced at the time of augmentation, a flapless, guided implant placement was performed (Figure 7).
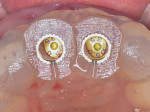
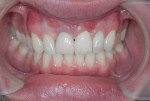
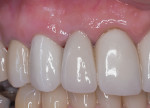
After approximately 10 weeks of osseointegration and soft-tissue maturation, restorative therapy was performed. Provisional restorations were placed to guide soft-tissue formation, and then cement-retained crowns were placed onto CAD/CAM abutments (Atlantis, Dentsply Sirona) (Figure 8).
Immediate implant placement offers an opportunity to expedite treatment, reduce the number of surgical interventions, and sometimes provide fixed provisional restorations. As with any treatment, proper diagnosis, treatment planning, and execution are needed for immediate implant therapy to be successful. Important factors to consider for predictable immediate implant treatment include implant selection, hard- and soft-tissue physiology, tissue contour management, provisionalization, and design of the definitive crown.9 Factors such as implant diameter and positioning in relation to the implant's orientation in the socket have significant ramifications on the esthetic outcome of immediate implant placement.10
Although the predictable osseointegration of immediately placed implants can be anticipated, resorption of the alveolar ridge at the site of extraction occurs despite implant placement.11,12 Simply placing an implant into a fresh extraction socket and temporizing it will not prevent continual remodeling and subsequent soft-tissue recession.13 As the evolution of immediate implant placement continues, methodology has changed, including steps such as flapless extractions and implant placement,14 grafting the gap between the implant and socket walls,15 and soft-tissue grafting.16
The osteoconductive properties of allografts and xenografts make them the most common materials for obturating the gap between the socket wall and implant. The soft-tissue graft most often used for augmentation around immediate implants is the subepithelial connective tissue graft. Although these grafts are highly successful, the morbidity associated with the procedure cannot be discounted. In many situations, soft-tissue allografts may serve as a viable substitute for autogenous grafts. Akimoto et al demonstrated this concept in a case series.17 Using a dermal allograft on the buccal aspect of a dental implant can increase soft-tissue thickness. The importance of the thickness of soft tissue cannot be overstated. Thicker mucosa is capable of masking underlying abutments18and preventing recession.19 It has also been demonstrated radiographically that augmenting naturally occurring soft tissue around implants with a dermal allograft can prevent crestal bone loss.20 Recently, the author published a technique article demonstrating a method of augmenting hard and soft tissue around immediately placed implants and providing screw-retained temporary crowns.21
Dermal Apron Technique
An example of the dermal apron technique is demonstrated in the following case. A 43-year-old female patient presented with a subgingival fracture of tooth No. 7 (Figure 9). After a flapless extraction was performed, the sockets were cleansed in the identical manner as in the previous case. Implant selection was based on several factors. First, the proper prosthetic platform should be provided for a physiologic emergence profile from the top of the implant through the soft tissues. Second, the diameter of the implant should not be too wide such that it would result in the implant contacting or being too close to the adjacent teeth or facial bone. A gap of at least 1 mm to 1.5 mm should exist between the implant and socket wall facially.22 The patient received a 3 mm x 13 mm implant to fulfill these criteria (Figure 10). This facilitated placement of a particulate bone graft into the gap. Implant surgery should provide a 1.5-mm to 2-mm thick buccal and lingual osseous plate, which has been shown historically to prevent soft-tissue recession.23
Management of this gap, therefore, plays a critical role in preserving contours of the ridge. The author prefers a composite bone graft consisting of mineralized allograft bone (FDBA, Symbios) and deproteinized bovine bone mineral (DBBM) (Bio-Oss®, Geistlich Biomaterials, geistlich-na.com). The ratio is approximately four parts FDBA and one part DBBM. Because implant therapy must achieve osseointegration, substitution of the bone graft is critical, and the allograft fulfills this role. Long-term space maintenance provided by the extremely slow replacement of the mineralized xenograft is also important to prevent soft-tissue recession, which ensues after hard tissue remodels.
As occurred in this case, soft-tissue augmentation is accomplished with the use of a dermal allograft, achieved via the dermal apron technique (Figure 11). The dermal allograft (Symbios PerioDerm) has a thickness of 0.4 mm to 0.8 mm. The orientation of the material is such that the basement membrane surface is adapted against the external surface of the facial bone and the connective tissue surface is approximated against the periosteum of the facial mucosa. This requires preparation of a small subperiosteal pouch, about 5 mm to 8 mm in depth, immediately before insertion of the allograft material. After the abutment screw of the provisional crown is tightened, a radiograph is obtained to confirm complete seating before sealing the access channel and suturing. As it was in this case, the temporary crown is always placed out of occlusal contact with the opposing teeth (Figure 12).
The patient was instructed to avoid all mastication in the operated area for about 6 weeks. A healing period of approximately 10 to 12 weeks precedes the initiation of restorative therapy. This allows for osseointegration and maturation of the peri-implant mucosa. At that point, a cement-retained crown is fabricated and seated onto a custom abutment (Figure 13).
Conclusion
Simply placing implants into available bone is often inappropriate for modern dental implant therapy to be successful. Implants must be placed in prosthetically guided positions and surrounded by stable hard and soft tissues. This often requires regenerative surgery either before or simultaneous with implant placement. With esthetic requirements involving not only the color, translucency, and character of the restoration, but also the contours, levels, and health of the surrounding tissues, increased emphasis is placed on the surgical aspects of implant therapy.
Regenerative treatment should be aimed at the restoration or preservation of pre-extraction alveolar ridge contours. In edentulous segments of the dentition, this may necessitate multiple procedures to rebuild hard and soft tissues. The use of recombinant growth factors and osteoinductive bone grafts make this possible in many situations.
When extracting hopeless teeth, often the infection associated with these teeth results in significant damage to the hard and soft tissues. Regenerative therapy initiated at the time of extraction can often reconstitute these tissues so they are capable of framing implant-supported restorations in the future. Selection of the appropriate graft materials and membranes and soft-tissue management are crucial factors. Biomaterials should be capable of supporting new tissue growth without causing inflammatory reactions and complications. They also should be substituted with viable bone and mucosa and not interfere with the long-term integration of dental implants.
Immediate implant therapy provides a unique opportunity for clinicians to expedite treatment. Immediately replacing a hopeless tooth with an implant capable of supporting a provisional restoration may result in greater patient satisfaction and enable the surgeon to utilize regenerative techniques that can preserve and even augment existing tissues. Bone grafts and allogeneic soft-tissue grafts reduce the morbidity associated with harvesting autogenous bone and mucosa. Current techniques, such as the dermal apron, can provide long-lasting, esthetic, and healthy outcomes for patients.
About the Author
Barry P. Levin, DMD
Clinical Associate Professor, Department of Graduate Periodontology, University of Pennsylvania, Philadelphia, Pennsylvania; Diplomate, American Board of Periodontology; Private Practice, Jenkintown, Pennsylvania
References
1. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87-91.
2. Misch CM. Bone augmentation of the atrophic posterior mandible for dental implants using rhBMP-2 and titanium mesh: clinical technique and early results. Int J Periodontics Restorative Dent. 2011;31(6):581-589.
3. Levin BP. Horizontal alveolar ridge augmentation: the importance of space maintenance. Compend Contin Educ Dent. 2001;32(8):12-21.
4. Levin BP. Alveolar ridge augmentation: combining bioresorbable scaffolds with osteoinductive bone grafts in atrophic sites. A follow-up to an evolving technique. Compend Contin Educ Dent. 2013;34(3):178-186.
5. Levin BP, Rubinstein S, Rosenthaler H, et al. Advanced surgical and restorative therapies aimed at rehabilitation of a severe dentoalveolar defect in the esthetic zone. J Implant Advanced Clin Dent. 2013;5(9):17-27.
6. Choquet V, Hermans M, Adriaenssens P, et al. Clinical and radiographic evaluation of the papilla level adjacent to single-tooth dental implants. A retrospective study in the maxillary anterior region. J Periodontol. 2001;
72(10):1364-1371.
7. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
8. Zubery Y, Nir E, Goldlust A. Ossification of a collagen membrane cross-linked by sugar: a human case series. J Periodontol. 2008;79(6):1101-1107.
9. Levin BP, Rubinstein S, Rose LF. Advanced esthetic management of dental implants: surgical and restorative considerations to improve outcomes. J Esthet Restor Dent. 2015;27(4):224-230.
10. Le BT, Borzabadi-Farahani A, Pluemsakunthai W. Is buccolingual angulation of maxillary anterior implants associated with the crestal labial soft tissue thickness? Int J Oral Maxillofac Surg. 2014;43(7):874-878.
11. Araujo MG, Sukekava F, Wennstrom JL, Lindhe J. Ridge alterations following implant placement in extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32(6):645-652.
12. Sanz M, Cecchinato D, Ferrus J, et al. A prospective randomized-controlled clinical trial to evaluate bone preservation using implant with different geometry placed into extraction sockets in the maxilla. Clin Oral Impl Res. 2010;21(1):13-21.
13. Kan JY, Rungcharassaeng K, Lozada JL, Zimmerman G. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 2011;26(1):179-187.
14. Tarnow DP, Chu SJ, Salama MA, et al. Flapless postextraction socket implant placement in the esthetic zone: part 1. the effect of bone grafting and/or provisional restoration on facial-palatal dimensional changes-a retrospective cohort study. Int J Periodontics Restorative Dent. 2014;34(3):323-331.
15. Cardaropoli D, Gaveglio L, Gherlone E, Cardaropoli G. Soft tissue contour changes at immediate implants: a randomized controlled clinical study. Int J Periodontics Restorative Dent. 2014;34(5):631-637.
16. Kan JY, Rungcharassaeng K, Morimoto T, Lozada J. Facial gingival tissue stability after connective tissue graft with single immediate tooth replacement in the esthetic zone: consecutive case report. J Oral Maxillofac Surg. 2009;67(suppl 11):40-48.
17. Akimoto KM, Schuler RF. Ridge width alteration after implant placement into the fresh extraction socket with deproteinized bovine bone mineral and acellular dermal matrix. Clin Adv Periodontol. 2012;2(2):89-95.
18. Jung RE, Holderegger C, Sailer I, et al. The effect of all-ceramic and porcelain-fused-to-metal restorations on marginal peri-implant soft tissue color: a randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2008;28(4):357-365.
19. Nisapakultorn K, Suphanantachat S, Silkosessak O, Rattanmongkolgul S. Factors affecting soft tissue level around anterior maxillary single-tooth implants. Clin Oral Implants Res. 2010;21(6):662-670.
20. Linkevicius T, Apse P, Grybauskas S, Puisys A. Influence of thin mucosal tissues on crestal bone stability around implants with platform switching: a 1-year pilot study. J Oral Maxillofac Surg. 2010;68(9):2272-2277.
21. Levin BP. The dermal apron technique for immediate implant socket management: a novel technique. J Esthet Restor Dent. 2016;28(1):18-28.
22. Misch CM. Bone augmentation of the atrophic posterior mandible for dental implants using rhBMP-2 and titanium mesh: clinical technique and early results. Int J Periodontics Restorative Dent. 2011;31(6):581-589.
23. Spray JR, Black CG, Morris HF, Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol. 2000;5(1):119-128.