You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
History of Ultrasonic Use in the Dental and Dental Hygiene Setting
Over 50 years ago, in 1952, US patent number 2,580,716 was issued to Lewis Balamuth. Entitled “Method and Means for Removing Material from a Solid Body,” it represented one of the most important developments in introducing ultrasonics to the field of dentistry.1 Originally designed as a cavity preparation procedure utilizing ultrasonic vibrations and an abrasive slurry, the new approach did not catch on readily with dental practitioners for a variety of reasons. Among them were visibility issues, slow cutting time, dulling of working tips, and, perhaps most importantly, the introduction of high-speed rotary cutting instruments.1,2 However, the demise of ultrasonic use in the restorative setting gave rise to a new era of calculus removal. When using the ultrasonic scaler with specialized tips and no abrasive slurry, dental professionals discovered that is was possible to remove deposits from the teeth.1 Ultrasonic instrumentation was formally introduced to the field of periodontics in 1955 when Zinner published a paper in the Journal of Dental Research elucidating the results from previous studies on use of the instrument without an abrasive slurry.3,4 By the late 1950s, the first power scalers were being utilized for heavy supracalculus removal with favorable results. During the 1960s, these instruments were declared acceptable, effective devices for supracalculus and stain removal.5 Throughout the 1960s and into the 1970s, clinicians and patients were warming up to the idea of power scaling as the procedure was less fatiguing for operators and patients spent less time in the chair.
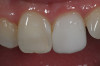
During the 1970s, researchers began investigating the causative nature of periodontal diseases. Dogma of the time suggested that periodontal disease was caused by mechanical irritants such as calculus. It was also believed that lipopolysaccharides or endotoxins derived from gram-negative bacteria were deeply embedded in the cementum.6,7 Treatment for periodontal inflammation was based on thoroughly removing calculus and rough cementum from tooth structures and planing root surfaces to a glassy-smooth texture.8 The “glassy-smooth” therapy endpoint often led to hourglass-shaped roots (Figure 1), hypersensitivity for the patient, and the occasional pulpal exposure.9
The 1980s ushered in a truly revolutionary time for ultrasonic instrumentation. One major drawback of early power scaling was the bulky nature of the insert tips which limited access to subgingival and interproximal areas.5,8 Clinicians were forced to primarily rely on Gracey curettes for debridement of periodontal pockets.6 Recognizing the potential for ultrasonic use in periodontal therapy, manufacturers began making and marketing thinner, probe-like tips designed specifically for subgingival debridement and curved tips developed for access to furcations. The newer, slimmer tips removed calculus safely and more efficiently than the earlier models, disrupted subgingival plaque biofilm, and detoxified the areas being treated.8 This improved technology in tip design allowed ultrasonic debridement to be compared favorably to periodontal hand instrumentation protocols.5,11-14
By the 1990s, researchers concluded that bacterial plaque was likely the culprit in periodontal inflammation and that, while calculus was a “contributing factor” due to its plaque-retentive characteristics, it was not the primary causative agent.9 In addition, clinical studies demonstrated that bacterial endotoxins were not as deeply embedded in cementum as originally thought. In fact, the cytotoxic bacterial by-product was loosely adherent to the root surface making rigorous root planing to remove the toxin unnecessary.8 It was determined that both ultrasonic and hand instrumentation could easily remove lipopolysaccharides while conserving cementum.5,12,13 Additionally, researchers found that while hand instrumentation did produce a significantly smoother root surface compared with ultrasonic debridement, there was no corresponding relationship in the reduction of periodontal inflammation between the two clinical protocols.5 Results of research endeavors shifted clinical endpoints away from producing traditional glassy smooth root surfaces to a gentler approach that eliminated subgingival bacteria, conserved tooth structure, and created a more biologically acceptable environment.15 At the same time, exciting research was being conducted by Wu and colleagues at the University of Washington (Seattle) that identified a cementum-derived protein that promotes the adhesion and spreading of periodontal cells. Named cementum attachment protein (CAP), the researchers discovered that CAP is not related to other collagens or attachment proteins and that it is a collagenous attachment protein localized in cementum. Their findings suggested that establishing true connective tissue attachment between root surfaces and alveolar bone might be related to the presence of CAP and furthered the notion that sparing cementum during instrumentation procedures would be beneficial.16 Rethinking clinical endpoints resulted in gradually replacing the term “root planing” with more representative descriptives such as “periodontal debridement,” “root detoxification,” and “nonsurgical periodontal therapy.”5,8 Based on the newly established therapy endpoints, the 1990s was a decade that welcomed ultrasonic use as a viable clinical option for periodontal debridement procedures. Dental hygiene clinicians were impressed by how favorably ultrasonic instrumentation compared to manual scaling procedures—virtually the same therapy outcomes could be achieved in less time with minimum operator fatigue, greater patient comfort, and decreased tooth surface loss.5,17-19
Today’s clinician relies on ultrasonic instrumentation for routine delivery of dental hygiene care. Faced with a plethora of different types of ultrasonic units and insert tips, it is incumbent upon dental hygiene practitioners to recognize differences in operation, clinical protocols, attributes and limitations associated with any given ultrasonic device. Understanding these differences is beneficial because the efficacy of powered instrumentation is dependent on the knowledge, skill, and technique of the operator using it.17 To that end, an overview of terminology is provided to aid the clinician’s understanding of the working dynamics of ultrasonic devices currently available.
Terms to Know
Acoustic streaming or turbulence: Believed to disrupt biofilm, acoustic streaming or turbulence refers to the swirling phenomenon created by the continuous vibration of an ultrasonic tip in a liquid environment.5,19
Amplitude: Sometimes referred to as “stroke,” amplitude relates to the distance the instrument moves during one cycle. Amplitude is influenced by the amount of electromagnetic power applied and can be regulated by the operator—the higher the power setting on an ultrasonic instrument, the greater the amplitude (Figure 2).1,5
Atomization action: The atomization action is the “halo effect” created as the irrigant flows along the ultrasonic tip toward the point and leaves the instrument in many directions (Figure 3).1
Cavitation: The phenomenon involved in producing atomization is cavitation. The term refers to the rapid formation and collapse of local bubbles or “cavities” within a liquid environment. The energy released against the tooth as a result of the implosion of the cavitational bubbles lyses bacterial cell walls and disrupts the microbial environment.1,5,8
Frequency: Ranging from 18,000 KHz to 50,000+ KHz, frequency is the number of times an instrument tip vibrates per second and is measured in kilohertz units. When a magnetostrictive insert is marked 30 KHz, for example, it means the tip will vibrate at 30,000 cycles per second (cps). While the majority of modern ultrasonic units are auto-tunable, manually tuned ultrasonic instruments are available which allow the operator to control the number of vibrations (frequency) of the ultrasonic unit.17
Irrigant or Coolant: The heat generated by ultrasonic tips requires continuous use of an irrigant or coolant to minimize temperature increases and decrease damage to periodontal, dentinal, and pulpal tissues.20 Many ultrasonic units rely on water irrigation obtained through the dental operatory line while newer units have self-contained irrigation devices which allow the operator to integrate delivery of therapeutic agents, such as antimicrobials, during ultrasonic instrumentation.16
Lavage: The fluid flow of an irrigant through the insert creates a lavage effect which allows debris, bacterial by-products, and blood to be constantly flushed from the treatment site during ultrasonic therapy.5,8
Magnetostrictive effect: Discovered by Joule in 1847, magnetostrictive effect is based on the ability of a magnetic field to change the length of ferromagnetic materials.21
Piezoelectric effect: The piezoelectric effect was first described by the Curie brothers in 1880 and involves subjecting certain crystals to mechanical pressure or tension to create an electric charge. An alternating electrical current is created when the direction of the mechanical stress (tension) is reversed (compression).1,22
Point: The point of the ultrasonic tip is defined as the terminal end of the insert and is the most powerful surface on an ultrasonic insert (Figure 4).8
Types of Ultrasonic Units
Currently, the dental hygiene clinician can choose to use either a magnetostrictive or piezoelectric ultrasonic device for debridement procedures. Both magnetostrictive and piezoelectric units have a variety of inserts and tip designs available and demonstrate comparable efficiency in calculus removal (Figure 5 and Figure 6).16,23 Each type of ultrasonic has distinct advantages and treatment considerations that operators must familiarize themselves with prior to use.
Magnetostrictive Ultrasonic Scalers
Historically, magnetostrictive ultrasonic devices have been the most popular type of power scaler utilized in the United States.16 Magnetostrictive ultrasonic scalers operate by creating a rapidly oscillating active tip area that effectively removes deposits while functioning at a frequency commonly ranging from 25,000 cps to 42,000 cps.4 The magnetostrictive inserts are composed of a stack of metal strips (most common) or rod of ferromagnetic material capable of being magnetized.8 To generate the working action, an alternating electrical current is applied to a wire coil located in the instrument’s handpiece which, in turn, causes the stacked metal strip or ferrite rod of the magnetostrictive insert to rapidly vibrate. The magnetostrictive effect is transferred by a rod (or connecting body) to the tip.8 The motion of the tip is determined by it’s geometry but for tips with a single bend the motion is primarily in the plane of the bend.26 This type of motion allows all sides of the insert tip to be active, although, specific considerations of varying degrees of power associated with different aspects of the tip need to be clarified. As with all power scalers, the point of the insert tip is usually the most powerful and instrumentation with the insert point oriented at a right angle to the tooth surface should be avoided at all times to avoid damage to tooth structures.8 Second most active is the concave (inner) surface of the stacked metal insert and the convex (back) surface of the insert tip. The least powerful surfaces are the lateral sides of the inserts. Keeping this in mind, the hygiene clinician can regulate energy dispersion and control patient sensitivity by selecting the appropriate insert surface to use during treatment.8
A coolant must be utilized when employing magnetostrictive ultrasonic instrumentation to dampen the heat that is automatically generated from the inserts. Maintaining a flow of irrigant that controls heat while minimizing aerosol production is key to the effective use of a magnetostrictive ultrasonic device. Clinicians need to keep in mind that increasing the power setting also increases aerosol formation which results in reduced coolant and cavitational effects leading to patient sensitivity.24
The decrease in cavitational effects also adversely affects ultrasonic detoxification of subgingival plaque leading to a less than ideal clinical outcome.24 It has been suggested that medium power settings not exceeding 50% capacity be utilized for moderate to heavy deposit removal while lower power settings are recommended for de-plaquing and removing biofilm and smear layer components.8,25 When heavier, tenacious deposits are encountered, increasing the power setting while using a standard, thicker tip insert will aid the hygiene clinician in removal. The standard insert has more bulk and surface area to handle the increase in power and is able to remove calculus with relative ease.15 An important clinical consideration to keep in mind is that as the diameter of the insert decreases so does the power requirement. For example, thin tips designed specifically for periodontal subgingival and furcation debridement procedures should only be used on lower power settings to minimize tip breakage and patient discomfort due to the increase in amplitude produced by a higher power setting.8
Piezoelectric Ultrasonic Scalers
Piezoelectric technology has been extremely popular in Europe and Asia for many years and numerous dental hygienists in the United States are discovering the benefits of using piezoelectric instrumentation.16 Piezoelectric power scalers utilize alternating electrical currents applied to reactive crystals in the handpiece to achieve the piezoelectric effect.1 Again this action is transferred by a rod to the tip where it translates to a working motion. While all tip surfaces are active, the lateral surfaces of the insert tip are the most comfortable for the patient to use in debridement procedures.4,8,16 Maintaining lateral instrument tip orientation makes piezoelectric debridement procedures a bit more technique-sensitive for most clinicians but many hygienists report that they feel they do not have to work as hard to remove deposits.8 As with all types of ultrasonic units, the point of the insert tip should not be oriented perpendicular to the tooth surface in order to minimize damage.
Generally operating between 25,000 cps and 50,000 cps, piezoelectric devices generate little heat in the handpiece. While it is advantageous to utilize an irrigant in order to reduce tip friction against the tooth surface, promote cavitation and provide beneficial lavage to the periodontal tissues, the irrigant flow can be minimized.16 Many piezoelectric as well as magnetostrictive ultrasonic instruments are manufactured to accommodate antimicrobial irrigation during operation (Figure 7). At the time this article was written, only two companies (Parkell, www.parkell.com; and Acteon, www.acteongroup.com) offered antimicrobial irrigants for ultrasonic instruments. Piezoelectric units require less irrigant use during instrumentation when compared to magnetostrictive units which require more irrigation liquid for cooling purposes.
In comparison to magnetostrictive inserts, piezo-electric tips are much smaller, allowing for easier storage and potentially lower cost (Figure 8).8 Like magnetostrictive inserts, there are many piezoelectric tip design options for operators to choose from. The design diversity allows hygienists to select the most appropriate tip to meet the clinical needs at hand. Most piezoelectric instruments require a torque device to secure and remove the tips from the handpiece which can be disruptive to clinicians requiring multiple tip options during debridement procedures.16
One unique feature of the piezoelectric scaler that dental hygiene practitioners should keep in mind is that, unlike magnetostrictive instruments, little magnetic field is generated at the handpiece when they are being used in the practice setting. This reduces the possibility of interference with other devices such as pacemakers. For the last several years, however, implanted pacemakers have been shielded and pose virtually no risk to the pacemaker patient receiving ultrasonic debridement therapy. The American Heart Association advises patients with artificial pacemakers to inform their healthcare providers that they have received the implant but identifies dental equipment as “device(s) with little or no risk.”27 It is prudent to err on the side of safety when in doubt as to the type of pacemaker a patient has received. In these cases, selecting a piezoelectric instrument is a good option for dental hygiene practitioners.
Clinician and Patient Considerations for Ultrasonic Instrumentation
The majority of periodontal experts currently believe that the best clinical results for nonsurgical periodontal therapy are attained by employing a combination of ultrasonic and hand instrumentation.5,6,10,11,28 Most dental hygienists agree that hand instrumentation has its place in rendering care but are relying more on powered scaling in the clinical setting for a variety of reasons. It has been well documented that ultrasonic instrumentation is not only as effective as hand instrumentation in debridement procedures but offers distinct advantages when used by skilled therapists.
The positive effects of lavage and cavitation offered by ultrasonic debridement are numerous. When compared to hand scaling, generated lavage and minimal gingival trauma associated with ultrasonic use results in reduced healing time after periodontal debridement therapy compared to hand scaling.8 Not only do power scalers disrupt and remove subgingival biofilm from the root surfaces and areas of periodontal pocketing, the cavitational and acoustic streaming effects disrupt plaque colonies slightly beyond the tip of the activated tip, making it an ideal therapy option for areas of difficult access.5,6,29 Access is further enhanced and shown to be superior to hand instrumentation when thin ultrasonic inserts are used to debride areas of Class II and III furcations as well as other deep narrow defects.8,31
With respect to time, ultrasonic debridement was demonstrated to be much quicker when compared to manual instrumentation, resulting in less operator fatigue.11,18, 30, 32 In addition to spending less time in the operatory receiving dental hygiene care, patients are pleased that powered scaling also creates less tissue distension and requires much less lateral force against the tooth surface for deposit removal resulting in increased comfort.5
Minimizing the need for lateral force to remove calculus benefits clinicians by reducing the risk of developing carpal tunnel syndrome and other musculoskeletal disorders.5,6,13 In fact, too much lateral force applied during instrumentation will decrease the effectiveness of the powered scaler by dampening the vibration of the ultrasonic device and may cause significant damage to the root surface.1
While the benefits of power scaling to patients and dental hygienists are numerous, there are some cautionary considerations associated with ultrasonic instrumentation. One concern is with aerosol production during the use of these devices. As previously mentioned, less irrigant is necessary to operate a piezoelectric unit than is required for operation of a magnetostrictive ultrasonic scaler. A study by Timmerrman and colleagues showed that atmospheric microbial contamination was negligible when using a piezoelectric power scaler.33 However, both types of devices generate an aerosol during operation. The generation of high levels of contaminated aerosols can be lessened by adjusting irrigant flow to the lowest amount necessary to optimally operate the ultrasonic unit. Additionally, having the patient use a pre-procedural antimicrobial rinse has been shown to decrease bacterial counts in aerosols by more than 90%.5 As is the case with any elective dental or dental hygiene treatment, patients presenting for care who are at high risk for infection or have communicable diseases should not be treated until their health stabilizes. This is especially true for ultrasonic therapy as these devices have the potential to generate contaminated aerosols. It is important to note that clarification of exactly how pathogenic aerosols generated from ultrasonic instrumentation is needed, as past investigations have failed to elucidate clear evidence that aerosol and splatter resulting from the use of ultrasonic scalers are an infection control risk.34
Another area of concern for dental hygiene clinicians involves infection control when using an ultrasonic unit. Although the tips can be sterilized, the rest of the unit’s components may not be accessible to sterilization procedures. It has been recommended that clinicians look for ultrasonic devices that have handpieces and irrigant reservoir bottles that can be sterilized in order to increase infection control.5
Maximizing patient comfort while delivering periodontal debridement is of primary importance to the dental hygienist. Proper use of the ultrasonic instrument with regard to tip adaptation and selection, power setting, and irrigant flow will greatly minimize patient discomfort. Additionally, the operator should avoid areas of hypersensitivity and demineralization while instrumenting with an ultrasonic device to prevent discomfort and permanent damage to tooth structures.5
Conclusion
Appreciating the operating concepts and treatment considerations of ultrasonic instrumentation greatly enhances the ability of dental hygiene care providers to maximize patient therapy outcomes. It is incumbent upon today’s dental hygienist to understand the attributes and limitations afforded by different types of ultrasonic instruments to make the best use of power scaling. As an integral component of debridement procedures, ultrasonic instrumentation provides treatment options that can be individualized to meet the periodontal needs of the dental hygiene patient.
Acknowledgments
With gratitude, the author wishes to acknowledge Mary D. Cooper, RDH, MEd, Tree Mainella, and Mike Reynolds for their assistance in securing historical literature and generating images for this article.
References
1. Clark SM. The ultrasonic dental unit: A guide for the clinical application of ultrasonics in dentistry and dental hygiene. J Perio. 1969;40(11):621-629.
2. Hughes C. Power-driven scalers: A review for practitioners. Dentistry Today. January 2008. Available at: www.dentistrytoday.net/ME2/Segments/Publications/Print.asp?Module=Publications. Accessed on September 21, 2009.
3. Zinner DD. Recent ultrasonic dental studies, including periodontia, without the use of an abrasive. J Dent Res. 1955;34:748-749.
4. Cooper MD, Wiechmann L. Essentials of Dental Hygiene: Clinical Skills. Chapter 3: Power-driven scaling. Upper Saddle River, NJ: Pearson Prentice Hall. 2006;83.
5. Bennett BL. Using power scaling to improve periodontal therapy outcomes. Contemporary Oral Hygiene. June 2007;14-21.
6. Nield-Gehrig JS. Fundamentals of Periodontal Instrumentation and Advanced Root Instrumentation. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2007:56-78.
7. Hughes FJ, Smales FC. Attachment and orientation of human periodontal ligament fibroblasts to lipopolysaccharide-coated and pathologically altered cementum in vitro. Eur J Prosthodont Rest Dent. 1992;2:63-68.
8. Carr M. Ultrasonics. Access. May-June 1999(special supplemental issue);1-8.
9. Walters C. Periodontal debridement techniques. Dental Teamwork. 1996;9(3):12-14.
10. Arabaci T, Cicek Y, Canackci CF. Sonic and ultrasonic scalers in periodontal treatment: A review. Int J Dent Hyg. 2007;5:2-12.
11. Tunkel J, Heinecke A, Flemming TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Supplement 3):72-81.
12. Hallmon WW, Rees TD. Local anti-infective therapy: Mechanical and physical approaches. A systematic review. Ann Periodontol. 2003;8:99-114.
13. Cooper MD, Mann NK. Instrumentation for root debridement. Contemporary Oral Hygiene. 2007;7:30-45.
14. Peterson CA, Lutz ER, Mauriello SM. A task analysis for ultrasonic instrumentation. J Pract Hygiene. 1995;4(2):11-15.
15. Wu D, Ikezawa K, Parker T, et al. Characterization of a collagenous cementum-derived attachment protein. J Bone Miner Res. 1996;11(5):686-692.
16. Carr MP, Bray KK. Update on ultrasonics. Dimensions of Dental Hygiene. May 2004;22-29.
17. Drisko CL, et al. Position paper: Sonic and ultrasonic scalers in periodontics. Research, Science and Therapy Committee of the American Academy of Periodontology. J Periodontol. 2000;71:1792-1801.
18. Cobb CM. Clinical significance of non-surgical periodontal therapy: An evidenced-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(supplement):6-16.
19. Walmsley AD, et al. Effects of cavitational activity on the root surface of teeth during ultrasonic scaling. J Clin Periodontol. 1990;17(5): 306-312.
20. Nicoll BK, Peters RJ. Heat generation during ultrasonic instrumentation of dentin as affected by different irrigation methods. J Periodontol. 1998;69(8):884-888.
21. Joule JP. On the effects of magnetism upon the dimensions of iron and steel bars. London, Philadelphia Magazine & Journal of Science (Series 3). February 1847;30:76.
22. Sweeney WT. Characteristics of ultrasonic vibrations. J Am Dent Assoc. December 1957;55:819.
23. Clinician’s Guide to Dental Products and Techniques. Clinical Research Associates Newsletter. 2003;27:10.
24. Chiew SYT, et al. Assessment of ultrasonic debridement of calculus-associated periodontally-involved root surface by the limulus amoebocyte lysate (LAL) assay: An in vitro study. J Periodontol. 1991;18:240-244.
25. Chapple ILC, et al. Effect of instrument power setting during ultrasonic scaling upon treatment outcome. J Periodontol. 1995;66(9):756-760.
26. Menne AOL, et al. Vibration characteristics of oscillating scalers. Jour Dent Res. 73:Spec, Abstr. #2661, p434, Mar 94
27. American Heart Association: Pacemakers: AHA recommendations. Available at: www.americanheart.org/presenter.jhtml?identifier=4676. Accessed on October 7, 2009.
28. Shaklee R. A blended approach. Dimensions of Dental Hygiene. 2006;4:26-27.
29. O’Neill-Smith K. The use of ultrasonics for the disruption of subgingival plaque biofilm. J Prac Hyg. 2006;15:14-15.
30. Turchetta A. Simplifying scaling and root planing with ultrasonics. Academy of Dental Therapeutics and Stomatology (a division of PennWell Publications); 2008. Available at: http://www.ineedce.com/courses/1483/PDF/SimplifyingScalingandRoot.pdf. Accessed on October 7, 2009.
31. Mengel R, et al. An in vitro study of various instruments for root planing. Int J Periodont Rest Dent. 1997;17(6):592-599.
32. Bernie K. A users’ guide to tip and insert options. Contemporary Oral Hygiene. January 2004;14-16.
33. Timmerman MF, et al. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31:458-462.
34. Harrel SK. Are ultrasonic aerosols an infection control risk? Dimensions of Dental Hygiene. June 2008;20-26.