Use of Oxalate Strips in Dentistry: Overall Response and Case Studies With Recent and Longstanding Sensitivity
Chad J. Anderson, MS, DMD; Robert W. Gerlach, DDS, MPH; Gerard Kugel, DMD, MS, PhD; and Marco Ferrari, MD, DMD, PhD
Abstract
Practice-based research was conducted to assess professional in-office treatment of dentin hypersensitivity with a strip-based device as part of a recall visit. The study population came from two sources within the dental practice. The majority were recall patients with evident sensitivity that was observed during routine care, while others were patients with history of dentin hypersensitivity in dental records. Treatment consisted of a 1.5% oxalate gel on a polyethylene strip (Crest® Sensi-Stop™ Strips, Procter & Gamble) that was professionally applied directly to sensitivity sites. Sensitivity was measured clinically and perceptually prior to and immediately after treatment, and again 30 days later. A total of 27 adults received oxalate gel strip treatment, and analysis focused on tooth location (arch and position) to ascertain the feasibility of introducing professional strip treatment as part of routine care. Results demonstrated that patients with cervical dentin hypersensitivity were easy to identify, professional strip application was feasible at different tooth sites across both arches, and treatment resulted in both immediate and durable sensitivity effects over a 1-month period.
Dentin hypersensitivity represents one of the most common painful conditions that may be discovered during routine dental examination. The etiology is multicausal, and reviews implicate both inadequate and aggressive brushing, poor oral hygiene, certain periodontal procedures, diet, occlusion, and other factors.1,2 Sites with gingival recession are most commonly affected, and the putative etiology typically involves evaporative, tactile, or thermal mediated fluid flow in dentin tubules and subsequent nerve stimulation. Some common dental procedures, such as prophylaxis, may involve both direct tactile stimulation and use of an air/water syringe. In the absence of local anesthesia, such care may invoke the classic temporary and exaggerated painful response characteristic of dentin hypersensitivity.
Differential diagnosis may be advisable, as painful symptoms may be attributable to caries, fracture, restorative failures, or other factors.3 Treatment options are equally diverse and, apparently, without accepted standard interventions. In one recent practice-based survey, northwest US dentists reported averaging more than eight different options for treating dentin hypersensitivity.4 The most prominent treatments included fluorides, bonding, glutaraldehydes, oxalates, calcium phosphate, and others, plus diet/behavioral counseling. There was little apparent consensus, perhaps in part attributed to uncertain effectiveness, and, on average, clinicians in the survey reported regular use of approximately three different techniques.
Despite its prevalence and available treatment options, dentin hypersensitivity is sometimes undertreated in the contemporary dental practice. Various diagnostic, therapeutic, economic, and other factors, alone or in combination, likely contribute to decision-making around sensitivity treatment. At-home options may require both longer-term compliance and deferred evaluation until a subsequent recall visit. Alternatively, sensitivity care may be achieved via professional in-office treatment using one of the viable options for managing dentin hypersensitivity.5 At minimum, professional approaches such as these represent an opportunity to tie diagnosis, treatment, and evaluation directly to a single office visit, provided those interventions yield immediate sensitivity relief.
One notable treatment option involves the use of oxalates, which have shown merit in treating established dentin hypersensitivity via brush, tray, or rinse applications.6-8 Separately, use of adhesive tape has been reported as one option for application of fluorides to treat dentin hypersensitivity. This new oxalate strip delivery affords some of the same conveniences seen with whitening strips, and like those strips, may allow direct professional application at sensitive sites. Accordingly, new practice-based research was conducted to assess the feasibility of in-office use of oxalate gel strips, and to assess the effects (if any) on immediate and durable sensitivity relief.
Methods
Practice-based research was conducted to ascertain the feasibility of in-office use of oxalate strips for patient care. Prior to initiation, the research proposal, investigator qualifications, informed consent, and recruitment plan were reviewed and approved by an independent human subjects review board. Eligibility was limited to adults with clinical evidence of sensitivity, preferentially individuals with air-related dentin hypersensitivity involving two teeth in different quadrants. Individuals were excluded because of pregnancy or nursing, severe periodontal disease, and/or fixed facial orthodontic appliances. Recruitment focused solely on patients-of-record, participation was voluntary, and no compensation was provided to study subjects as part of this research.
Three visits were planned: an optional screening visit, baseline measurements and treatment, and posttreatment follow-up approximately 30 days thereafter. Dental records were reviewed, and individuals with reported ongoing sensitivity were scheduled for screening (if needed). Most participants, however, were simply notified of the research after completion of recall maintenance visits if sensitivity was noted or reported as part of that routine care. First, consent, eligibility, oral examination, and intraoral photographs were collected. Sensitivity to air stimulation was then measured using a 1-second blast of cool air from a dental air/water syringe. After each stimulus, response was quantified using standard clinical and perceptual measures. For the clinical assessment, a trained dentist examiner used a standard four-point categorical severity scale ranging from unresponsive (0) to responsive/painful/discontinue (3). For the self-assessment, subjects recorded their perceived pain level using a continuous linear visual analog scale (VAS) display on a tablet application, with outcomes recorded as 0 to 100 based on increasing discomfort.
In-office treatment consisted of a 1.5% oxalate gel on a 10-mm x 30-mm polyethylene strip (Crest® Sensi-Stop™ Strips, Procter & Gamble, www.dentalcare.com) professionally applied directly to sensitivity sites. Per product labeling instructions, teeth do not need to be dried before application. Application took approximately 1 minute; it included removal of the strip unit from its foil pouch container, peeling of the strip from its attached backing liner, and professional placement of the gel side of the strip at the sensitive site(s). A timer was set for 10 minutes, and midway through, each strip was checked for retention and clinical intraoral photographs were taken. At the end of the treatment time (10 minutes from application), strips were professionally removed and discarded, posttreatment measurements were obtained, and subjects were reappointed approximately 30 days later for reevaluation. Because this research assessed strip use as part of routine patient care, there was no attempt to standardize at-home oral hygiene after baseline.
One clinician performed both the treatment and clinical assessment. The clinical and perceptual responses to air stimulus were collected independently to limit bias. Teeth were tested sequentially in ascending tooth number. At each visit, a clinical examination was performed to assess safety, and subjects were interviewed regarding any adverse events between visits.
Sensitivity scores from oxalate-treated teeth were analyzed (tooth-level response) and averaged (subject-level response), with the latter representing the primary response. For the subject-assessed response only, VAS was logit-transformed to meet assumptions of normality for analysis. Comparisons to baseline used paired difference t-tests with 5% levels of significance, and safety data were summarized.
Results
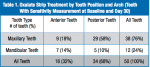
This practice-based research consisted of 25 women and 2 men ranging in age from 18 to 71 years (mean age = 45.1 years). Of these, the overwhelming majority (89%) had two sensitive teeth identified in different quadrants for testing. In total, 51 teeth were selected for strip application. One tooth was excluded from analysis due to an unrelated adverse event, yielding 50 teeth with pretreatment, immediate posttreatment, and 30-day recall assessments. Various tooth types were included in this practice-based assessment (Table 1).
Prior to treatment, the overall clinical air sensitivity Schiff Index mean (SD) was 2.4 (0.6). Schiff scores ranged from 1.0 to 3.0, and did not significantly (P > .66) differ by tooth type. The self-perceived air sensitivity VAS mean (SD) was 72.5 (11.8). VAS scores ranged from 22.0 to 100.0, and as with the clinical scores, perceptual sensitivity did not significantly (P > .23) differ by tooth type. Pretreatment clinical and perceptual scores were correlated (r = 0.51).
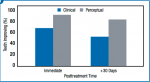
Strips were applied in-office for 10 minutes, after which, teeth were immediately retested for air-related sensitivity. The majority of teeth had measured improvements in both the Schiff Air Index and VAS when retested at both the immediate posttreatment and +30-day timepoints (Figure 1). Statistical analysis adjusting for subject (most participants had two teeth treated) demonstrated significant (P < .003) reductions in clinical and perceptual sensitivity versus baseline immediately after in-office treatment, and again 30 days thereafter. The overwhelming majority of subjects exhibited some improvement. Immediately after treatment, 81% to 96% of individuals had measured improvements in clinical or perceptual sensitivity. At the Day 30 posttreatment visit, 56% to 74% of subjects showed reduced sensitivity at one or more teeth.
While individual tooth responses varied, all tooth categories exhibited mean improvement relative to baseline. Analysis by location, adjusting for baseline sensitivity levels, showed mean improvements ranging from 21% to 40% for anterior and posterior teeth depending on timepoint and endpoint (Table 2). A similar analysis by arch, adjusting for baseline sensitivity levels and baseline-by-arch interactions, showed mean improvements ranging from 22% to 49% for maxillary and mandibular teeth (Table 3). Changes in sensitivity were well-correlated after 30 days, ranging from 0.54 to 0.80 based on location, and 0.38 to 0.78 based on arch.
For safety, there were three instances where strip application possibly contributed to temporary, contact-related tooth sensitivity. Each of these events was considered as mild in severity and did not impact successful completion of the 10-minute in-office application time for the sensitivity strip. In addition, one subject required endodontic therapy and was excluded from the analysis.
Discussion
Recent multipractice research found dental pain, in general, and dentin hypersensitivity, specifically, to be relatively common.10 The present authors interpret that research as a “rule of one-thirds,” wherein approximately one-third of adult practice patients have dental pain of some type, and of those, approximately one-third have dentin hypersensitivity. This new research identified sensitivity within patients-of-record in a contemporary California dental practice, many of whom were recruited at the time of routine recall prophylaxis without difficulty. Such patient types may be more or less common and/or obvious, depending on the nature of the practice. Applying the “rule of one-thirds” to the California practice in this research, such occurrence rates would translate to approximately 12 patients with dentin hypersensitivity each week.
Practice-based research has identified numerous options for treatment, with little consensus across offices or even within a single dental office.4 This was also the case in the California practice involved in this study, where nine different sensitivity products were available at the time of the research. While not used for sensitivity, previous research has identified the feasibility of professional strip application, for example, in the area of in-office tooth whitening.10 While such applications are typically limited to the anterior facial dentition, other concurrent research has extended usage to professional treatment of sensitivity, albeit within the framework of a university research clinic.11 This new research adds to the office-based treatment options, as it establishes the feasibility of direct professional application of sensitivity strips within a functioning dental practice. In this research, oxalate strips were applied and removed directly by a dentist. Various teeth were treated (typically two sites in different quadrants), with the majority of strips placed on posterior teeth. While professional (gloved) placement was more complicated in the posterior region, immediate clinical responses were noted in all areas of the mouth. Few of the preceding sensitivity options in the aforementioned California office have similar practice-based evidence involving both application and longer-term outcome assessment in current patients.
Several factors have been long recognized to contribute to the merits of practice-based research in dentistry.12 Practice-based research, for example, may provide access to different patient types and different evaluations than traditional clinical trials. There are, however, obvious limitations. Examiner blinding, for example, may be difficult or impossible to achieve for professionally administered treatments, and this may contribute bias to clinical measurements. As such, this new single practice-based study used a combination of clinical and subjective assessments specifically to limit bias. The oxalate strip treatment in this clinical trial yielded immediate sensitivity reductions, measured clinically by the dentist and subjectively by the patient. Most individuals had persistent relief for 1 month with uncontrolled at-home oral hygiene. Side effects were generally few in number, mild in severity, and with no treatment implications. While retreatment was not assessed in this research, it is a possible viable option with reevaluation at subsequent visits.
The following two case studies exemplify some of the challenges associated with diagnosis and treatment.
Case 1
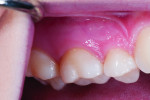
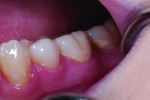
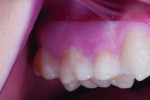
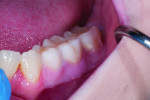
The first case involves a healthy, 19-year-old female patient with generalized sensitivity on the posterior teeth in all four quadrants and evident recession of 1 mm or less on the facial surfaces of molars (Figure 2 and Figure 3). Her medical history was unremarkable. With respect to dentistry, the patient presented with a history of tooth bleaching, recent restorative work unrelated to her sensitivity, and routine recall prophylaxis. Two posterior sites (maxillary right and mandibular left) were selected and measured for air-related sensitivity, and these contralateral regions were each treated in-office with a single oxalate gel strip for 10 minutes (Figure 4 and Figure 5). Responses were generally similar on each side of the mouth and showed appreciable sensitivity relief following treatment. For tooth No. 3, the clinical sensitivity score was 3 at baseline, 2 immediately after in-office treatment, and 1 at the 30-day recall, while for tooth No. 19, the measured responses were 3, 1, and 1, respectively. Patient perceived responses were comparable to those measured clinically. VAS results were 76, 54, and 3 for the maxillary right molar, and 88, 55, and 25 for the mandibular left molar.
Case 2
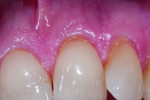
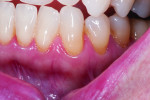
In the second case, a 62-year-old woman presented with longstanding thermal sensitivity in all areas of the mouth. The medical history included arthritis, temporomandibular pain, penicillin allergy, and antidepressant medications. Recent dental care was limited to maintenance. Malocclusion and recession were evident, with the latter involving most of the posterior facial dentition (Figure 6 and Figure 7). Two sites (maxillary and mandibular left) were selected for measurement, after which, two oxalate strips were professionally applied to cover the recession sites and adjacent gingiva (Figure 8). The mandibular cuspid site showed immediate and durable improvement in both clinical and subjective sensitivity assessments, while the maxillary central incisor site failed to exhibit a measurable response after one strip application.
Summary of Cases
Both cases involved recession-associated sensitivity. One case was of relatively recent origin (within a year), while the other was longstanding (more than a decade). Clinical presentation varied with respect to evident recession, illustrating the complexity involved in diagnosing dentin hypersensitivity. In each case, two strips were applied in-office for 10 minutes. One case had isolated areas of recession and treatment of contralateral posterior teeth, while the other had multiple sites with recession and sensitivity, and treatment was limited to the anterior dentition on one side of the mouth. These cases illustrate that different facial sites can be treated, though clinical application was easiest in the anterior region. While it was possible to cover multiple adjacent sites, coverage for each strip was limited to approximately three teeth.
Conclusion
The safety and effectiveness of 1.5% oxalate gel strips was tested in a clinical trial conducted in a dental practice setting to study professional application for sensitivity treatment under “real-life” conditions. The results of this study demonstrated that: (1) patients with cervical dentin hypersensitivity were readily identified within the practice as part of routine recall visits; (2) professional strip application was relatively easy to accomplish at all facial tooth sites in both arches; (3) use of a single oxalate strip resulted in an immediate reduction in sensitivity during the office visit that was evident to both the clinician and study subjects; and (4) treatment effects were generally durable over a 30-day posttreatment period without meaningful safety issues. Based on these outcomes, in-office use of 1.5% oxalate strips may represent a viable professional treatment for sensitivity that could readily be administered and evaluated during a single routine recall visit.
Acknowledgments
This clinical trial was undertaken as part of graduate research training at University of Siena, Siena, Italy, and conducted at Anderson Dentistry, Fresno, California. Melanie Miner and Lisa Sagel (both of Procter & Gamble) supported analysis and reporting.
Disclosure
This research was sponsored by Procter & Gamble.
About the Authors
Chad J. Anderson, MS, DMD
Private Practice
Fresno, California
PhD candidate
University of Siena
Siena, Italy
Robert W. Gerlach, DDS, MPH
Research Fellow
Global Oral Care R&D
Procter & Gamble
Mason, Ohio
Gerard Kugel, DMD, MS, PhD
Professor
Associate Dean for Research
Tufts University School of Dental Medicine
Boston, Massachusetts
Marco Ferrari, MD, DMD, PhD
Dean
University of Siena School of Dental Medicine
Siena, Italy
References
1. Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51(3):323-332.
2. Pashley DH. How can sensitive dentine become hypersensitive and can it be reversed? J Dent. 2013;41(suppl 4):S49-S55.
3. Addy M, Smith SR. Dentin hypersensitivity: an overview on which to base tubule occlusion as a management concept. J Clin Dent. 2010;21(2):25-30.
4. Cunha-Cruz J, Wataha JC, Zhou L, et al. Treating dentin hypersensitivity: therapeutic choices made by dentists of the northwest PRECEDENT network. J Am Dent Assoc. 2010;141(9):1097-1105.
5. West NX, Seong J, Davies M. Management of dentine hypersensitivity: efficacy of professionally and self-administered agents. J Clin Periodontol. 2015;42 suppl 16:S256-S302.
6. Pillon FL, Romani IG, Schmidt ER. Effect of a 3% potassium oxalate topical application on dentinal hypersensitivity after subgingival scaling and root planing. J Periodontol. 2004;75(11):1461-1464.
7. Pamir T, Dalgar H, Onal B. Clinical evaluation of three desensitizing agents in relieving dentin hypersensitivity. Oper Dent. 2007;32(6):544-548.
8. Sharma D, McGuire JA, Gallob JT, Amini P. Randomised clinical efficacy trial of potassium oxalate mouthrinse in relieving dentinal sensitivity. J Dent. 2013;41 suppl 4:S40-S48.
9. Lee SH, Lee NY, Lee IH. Clinical evaluation of the efficacy of fluoride adhesive tape (F-PVA) in reducing dentin hypersensitivity. Am J Dent. 2013;26(3):143-148.
10. Gerlach RW, Barker ML, McMillan DA, et al. In-use comparative kinetics of professional whitening strips: peroxide recovery from strips, teeth, gingiva, and saliva. Compend Contin Educ Dent. 2004;25(8 suppl 2):14-20.
11. Papas A, Singh M, Magnuson B, et al. Randomized controlled trial evaluating use of two different oxalate products in adults with recession-associated dentin hypersensitivity. Compend Contin Educ Dent. 2016;37(spec iss 1):26-31.
12. Curro FA, Grill AC, Thompson VP, et al. Advantages of the dental practice-based research network initiative and its role in dental education. J Dent Educ. 2011;75(8):1053-1060.