Comparative Performance of Two Whitening Systems in a Dental Practice
Abstract
OBJECTIVE: A randomized, controlled clinical trial was conducted to compare the safety and whitening efficacy of high-adhesion tooth-whitening strips with a marketed in-office professional tooth-whitening system. METHODS AND MATERIALS: This open-label study was conducted in a private dental practice, and adult volunteers were assigned (2:1) to either 9.5% hydrogen-peroxide whitening strips (Strip group) or an in-office light plus 25% hydrogen-peroxide whitening gel treatment (In-office group). The Strip group was instructed to treat the maxillary arch once daily for 30 minutes over a 20-day period, while the In-office group underwent professional application of light plus whitening gel in a single office visit. Whitening response was measured as change in yellowness (b*) and lightness (L*) at Day 21 using standardized digital images of the maxillary anterior teeth, while safety was assessed as tooth sensitivity and oral irritation occurrence. A total of 45 subjects enrolled, were randomized, and received treatment; 44 completed the study. RESULTS: At Day 21, significant improvement in b* and L* was noted in both groups (P ≤ 0.001). The adjusted mean (SE) ΔL* in the Strip group (1.72 [0.104]) was significantly greater than that in the In-office group (1.17 [0.153]) (P = 0.005). Both test products were well tolerated. Overall, both the strip and in-office treatments resulted in significant tooth whitening.
While peroxide has a lengthy history in dentistry, its use for tooth whitening gained in popularity following the introduction of the custom-tray approach, in which low peroxide concentrations were applied at-home overnight for several weeks.1 Peroxide concentration affects response, so other efforts focused on the use of higher peroxide concentrations. Examples included in-home-use trays or direct application during an office visit, sometimes with lights or other adjuncts to promote peroxide reactivity.2 Irrespective of the approach, these follow-on products were largely directed at reducing treatment time or improving whitening response compared with the initial overnight trays.
In 2000, a novel peroxide-based whitening strip was introduced to improve ease of use and access to tooth whitening. This technique, which used a flexible polyethylene strip to deliver a thin hydrogen-peroxide bleaching gel to the anterior dentition, reportedly offered advantages over other delivery systems in terms of overall peroxide dose, contact time, and convenience.3 In 2004, further research established the safe use of higher peroxide concentrations via a very thin gel to deliver improved strip-based whitening.4
Recently, a novel two-polymer, high-adhesion gel matrix was developed, with adhesive and cohesive properties to allow sustained delivery of hydrogen peroxide over longer time periods. A new clinical trial was conducted to compare the safety and tooth-whitening efficacy of this new, high-adhesive strip technology with a popular in-office, light-aided tooth-whitening system.
Methods
This clinical trial was conducted within an active dental practice to assess response among a population typical of contemporary dentistry. After institutional review, adult patients in the practice were recruited if they desired tooth whitening but had no history of previous whitening or current tooth sensitivity. In addition, subjects were excluded due to labeling restrictions with the in-office product that served as the experimental control in this study. After informed consent, enrolled subjects were assigned to the strip or in-office products, and whitening outcomes were measured instrumentally at two time points over a 3-week period.
Subjects were randomly assigned in a 2:1 ratio to the Strip or In-office groups, balancing groups on baseline tooth color and age. For the Strip group, each subject received 20 9.5% hydrogen-peroxide whitening strips, a timer, and written instructions to treat the maxillary arch for 30 minutes once daily at-home. For the In-office group, a light-cured resin was applied for tissue isolation, eye protection was placed, and subjects underwent a series of three continuous 15-minute treatments with a 25% hydrogen-peroxide gel and a 365 nM to 500 nM light. Both arches were treated, after which neutral fluoride (0.5% KNO3+0.22 NaF gel) was applied according to manufacturer’s instructions. Subjects in both treatment groups were provided an anti-cavity dentifrice and a soft, manual toothbrush for use during the study. After completion, subjects were supplied a marketed box of whitening strips.
Effectiveness was measured from digital intraoral images collected at baseline and after 21 days following a standard method that has been used in numerous clinical trials at various dental schools and other settings.5 A chin rest was used for alignment, and standard lighting was achieved with two side-mounted 150-watt lights. Images of the facial anterior tooth surfaces were captured with a high-resolution digital camera (JVC KY-F75U CCD, JVC Professional Products Co., https://pro.jvc.com), a 25-mm lens, and a linear polarizer to permit cross-polarized light. Image analysis was used to derive mean color scores from the blinded images of the six maxillary anterior teeth, and change in tooth color was determined by comparing scores at baseline and post treatment. Whitening was represented by a negative Δb* (reduced yellowness) and positive ΔL* (increased lightness), because these individual color parameters have been independently shown to correlate to self-perception of tooth whitening.6 Safety was assessed from intraoral examination and interview. Comparisons to baseline were investigated using a paired-difference t-test, while groups were compared using analysis of covariance. All comparisons were tested at a two-sided 0.05 level of significance.
Results
A total of 45 subjects (30 in the Strip group and 15 in the In-office group) enrolled and were treated, with 44 completing the 21-day study. Subjects ranged from 18 to 61 years, with a mean (SD) age of 37.6 (10.4) years; 62% were female. Subjects exhibited considerable variation in tooth color at baseline, with Δb* ranging from 14.0 to 21.3, and ΔL* ranging from 67.8 to 78.2. Groups were balanced with respect to demographic parameters (P > 0.78) and baseline tooth color values (P > 0.97).
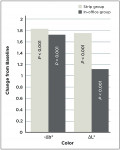
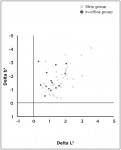
At Day 21, mean color improved significantly in both groups as indicated by a significant (P ≤ 0.001) reduction in yellowness and a significant (P ≤ 0.001) increase in lightness (Figure 1). As illustrated in the scatterplot (Figure 2), all 44 subjects had a measured two-parameter (Δb* and ΔL*) improvement in tooth color after treatment. Between-group comparison of adjusted mean changes from baseline showed a significant (P = 0.005) increase in lightness (ΔL*) in the Strip group (Table 1).
Tooth sensitivity and oral irritation were the symptoms most commonly reported by subjects. While tooth sensitivity was reported in a markedly higher percentage of subjects in the In-office group than the whitening Strip group (20% versus 3%), oral irritation was reported only by subjects in the Strip group (10%). Symptoms were generally mild, though one subject in the In-office group reported severe tooth sensitivity during treatment. Clinical examination at Day 21 was unremarkable, and no subject discontinued treatment early due to an adverse event.
Discussion
In this study, subjects were recruited from within a dental practice and then randomized in a 2:1 ratio to ensure that the sample size was sufficient for evaluation of the new whitening strips. A popular in-office treatment was selected as an experimental control for the study. Response was measured after 21 days, because this coincided with the day after scheduled completion of strip treatment, and because the authors believed it would be after any appreciable relapse that has been reported following light-aided, in-office whitening.7 In this study, both the whitening strip and the in-office treatments resulted in significant (P ≤ 0.001) tooth color improvement relative to baseline. Furthermore, all subjects (100%) exhibited objective color improvement after treatment either with whitening strips at-home or gel plus light combination in-office.
While both treatments were effective, comparative Day 21 whitening response favored repeated at-home treatment, with the 9.5% hydrogen-peroxide high-adhesion strips exhibiting significantly greater (P ± 0.005) lightness improvement (ΔL*) and directionally greater yellowness reduction (Δb*) relative to the in-office system. Both test products were generally well tolerated. Subjects in the In-office group experienced a higher frequency of tooth sensitivity, but no evident oral irritation (reported or observed). The latter could be attributed to the tissue isolation that occurred prior to the application of the 25% hydrogen-peroxide gel, or the Day 21 timing of the post-treatment examination. As such, results from this dental practice study are consistent with a larger clinical trial meta-analysis that implicated light use during whitening as contributing to a significant increase in tooth sensitivity.8
The research demonstrates limitations in conducting clinical trials in a practice setting. For example, in order to minimize visits, the study compared products at a common time point (Day 21) and different “relapse” times (Day 21 was 1 post-treatment day for the Strip group, and 20 post-treatment days for the In-office group). Previous research with instrumental methods measured a 45% to 52% post-treatment relapse in Δb* and ΔL* following one in-office treatment, while whitening strips were shown to relapse by only 1% to 4% over a longer period.7,9 Day 21 was selected as the only post-treatment evaluation to minimize office recall visits, with the expectation that both the strip and in-office systems would have equilibrated with respect to color at that time. Other time points, earlier or later, may yield different results with respect to comparative efficacy or safety.
This was a true hypothesis testing clinical trial comparing whitening strips to a professionally administered whitening system and, as such, differs from observational or other research that may be more common within a single dental practice. Because there was limited evidence on single practice outcomes with the strips or in-office system, the research followed accepted standards and did not presume that either treatment would be effective in this specific setting. The endpoints (Δb* and ΔL*) were selected a priori because these instrumental outcomes were previously established to be well correlated to first-person assessment of whitening improvement and, hence, the personal relevance of any measured whitening response in this dental practice.6 In addition, the two-parameter (Δb* and ΔL*) responses were used because these outcomes provided easily interpretable data on whitening consistency or inconsistency as measured for each subject in the study.9 Since both treatments resulted in whitening, use of other directional endpoints like Δa* or directionless composites like ΔE would have yielded similar responses but without the by-subject whitening consistency seen with Δb* and ΔL*.
This research establishes the feasibility of conducting objective whitening evaluations within a functioning dental practice. The authors believe there are potential advantages pertaining to relevance, and disadvantages pertaining to logistics, with clinical trials of this nature. One such limitation involves blinding, particularly since one group received a professionally administered in-office treatment, while the other received whitening strips for at-home use. The physical constraints of a dental practice can readily impact on maintenance of study blinding. Since this research was open-label, the authors used an instrumental, objective method (digital image analysis) with archival output, because this method had previously been reported to have merit in limiting any unintentional bias in outcome measurement with dissimilar products.10
Summary
In this dental practice research, both the high-adhesion 9.5% hydrogen-peroxide whitening strips and 25% hydrogen-peroxide light-aided in-office system yielded significant post-treatment tooth color improvement. Subjects in the Strip group experienced significantly greater improvement in tooth lightness compared with the In-office group at Day 21, and both treatments were generally well tolerated.
DISCLOSURE
This study was supported in part by The Procter & Gamble Company, Mason, Ohio.
ABOUT THE AUTHORS
Ronald Perry, DMD, MS
Professor, Director of the Gavel Center for Restorative Research, Tufts University School of Dental Medicine, Boston, Massachusetts
Erinn Conde, BS
Clinical Trial Manager, The Procter & Gamble Company, Mason, Ohio
Svetlana Farrell, DDS, PhD
Section Head, Clinical Research for Oral Care, The Procter & Gamble Company, Mason, Ohio
Robert W. Gerlach, DDS, MPH
Research Fellow in Worldwide Clinical Investigations, The Procter & Gamble Company, Mason, Ohio
Jennifer Towers, MS
Director of Dental Research, Tufts University School of Dental Medicine, Boston, Massachusetts
REFERENCES
1. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-176.
2. Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006;34(7):412-419.
3. Sagel PA, Odioso LL, McMillan DA, Gerlach RW. Vital tooth whitening with a novel hydrogen peroxide strip system: design, kinetics, and clinical response. Compend Contin Educ Dent Suppl. 2000;(29):S10-S15.
4. Gerlach RW, Sagel PA. Vital bleaching with a thin peroxide gel: the safety and efficacy of a professional-strength hydrogen peroxide whitening strip. J Am Dent Assoc. 2004;135(1):98-100.
5. Sagel PA, Gerlach RW. Application of digital imaging in tooth whitening randomized controlled trials. Am J Dent. 2007;20 Spec No A:7A-14A.
6. Gerlach RW, Barker ML, Sagel PA. Objective and subjective whitening response of two self-directed bleaching systems. Am J Dent. 2002;15 Spec No:7A-12A.
7. Kugel G, Ferreira S, Sharma S, et al. Clinical trial assessing light enhancement of in-office tooth whitening. J Esthet Restor Dent. 2009;21(5):336-347.
8. He LB, Shao MY, Tan K, et al. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J Dent. 2012;40(8):644-653.
9. Yudhira R, Peumans M, Barker ML, Gerlach RW. Clinical trial of tooth whitening with 6% hydrogen peroxide whitening strips and two whitening dentifrices. Am J Dent. 2007;20 Spec No A:32A-36A.
10. Gerlach RW, Zhou X. Comparative clinical efficacy of two professional bleaching systems. Compend Contin Educ Dent. 2002;23(1A):35-41.