Increasing Lateral Ridge Width for Implant Placement Using Calcium Sulphate Bone Cement Technique
David Baranes, DDS; Gregori M. Kurtzman, DDS; and Robert A. Horowitz, DDS
ABSTRACT
Dental implant practitioners are often faced with a presentation of a narrow crest, making it difficult or impossible for implant placement and requiring modification of the ridge to allow implants to be utilized. Many different factors need to be considered when choosing the technique to expand the ridge laterally. Ideally, the technique should facilitate the goal of ridge width increase while reducing inflammation and postoperative pain for the patient. In this study, patients were treated for lateral ridge augmentation to improve ridge width using a calcium sulphate-based bone cement with a protocol that does not require membrane placement at the time of grafting. This minimally invasive, relatively economical surgical technique is aimed at improving postoperative comfort for patients while permitting widening of the lateral ridge.
Frequently, in the maxilla and mandible following long-term tooth loss or extraction when significant periodontal disease is present, changes to osseous dimensions are noted. A narrow crest may be located in an area limited to one or two teeth, or in larger areas ranging from a quadrant to the full arch.1 The cause of this change may be related to periodontal disease, traumatic injury, or simply the physiologic anatomy of that particular patient. These osseous changes occur in both the vertical and horizontal dimensions. Thus, osseous grafting to regain adequate bone to place and maintain implants or to restore ridge contours becomes clinically essential for esthetic and functional reasons.
Ridge augmentation becomes necessary, especially when implant placement is in the treatment plan to replace those missing teeth. Various techniques have been developed and advocated to improve ridge width and are generally referred to as guided bone regeneration. These techniques have been widely described in the literature and documented with predictable clinical success in the treatment of narrow ridges.2 However, these techniques have entailed complex surgical procedures with long healing phases and a risk of membrane exposure during the initial healing period, along with possible subsequent loss of some or all of the bone, osseous graft material, and/or exposure of threads on immediate socket implants.3-6 Use of a membrane, required with many osseous graft materials, also increases treatment costs related to the membrane and its fixation devices, such as screws and tacks.
The use of autogenous blocks of intraoral or extraoral origin has also been an alternative to particulate osseous graft materials of either autogenous, allograft, xenograft, or synthetic origin when augmenting the narrow ridge.7,8 Nevertheless, this technique has several serious drawbacks, including the risk of complication at the donor site and the incidence of morbidity or paresthesia when the harvesting is from the ramus area. There may be longer healing periods then with particulate grafts. This brings a significantly higher treatment fee related to the more complex surgery required as well as a longer duration of surgery and supplemental materials needed.9-12
Ridge splitting is an alternative that is not always feasible when the ridge width is very narrow or consists of only cortical bone with no cancellous bone between the buccal and lingual plates.13 If the surgery is not performed well, significant damage to the residual ridge may result.14
As mentioned, particulate osseous graft materials can include autograft (autogenous bone from the same patient), allograft (human bone from another individual), xenograft (materials from different species), or alloplast (synthetic materials). These have all had long-term reported use and each has benefits and potential issues depending on the literature read and reviewed.15-20
Calcium sulphate is used in orthopedic surgery; it is a material of choice for traumatic bone augmentation due to its osteoconductive, bacteriostatic properties.21,22 It is fully resorbable, being replaced by the host bone over time, allowing space maintenance while migration of host osteoblasts and angiogenesis occurs. Within orthopedic surgery, it has been reported that the use of calcium sulphate to treat bone deficits found that the periosteum did not infiltrate the graft material.23,24
Bond Apatite® (Augma Biomaterials Ltd, augmabio.com) is a bone cement consisting of two-thirds biphasic calcium sulphate and one-third synthetic hydroxyapatite crystals of various sizes ranging from 90 microns to 1 mm.6 Intraorally, the material is in the form of a cement that forms a compact, solid block that is adherent to the underlying host bone. Histologically, angiogenesis is promoted to transform 90% of the graft into the patient's bone.25,26 Moreover, it does not permit epithelial cells in the overlying soft tissue to penetrate into the graft as it organizes during the healing and maturation phases.20,21 Yet, it allows the soft tissue to proliferate over the surface, closing any minor gap at the incision line and negating the need for a membrane to cover any minor exposed graft material.
The Bond Apatite protocol has three clinical consequences: the flap must be of full thickness so that the periosteum is in contact with the graft material; the flap must be closed, stretched, and stable over the underlying graft material, and flap closure may allow up to a 2 mm to 3 mm gap between the flap margins at the incision without compromise to the intended result; and, thus, this bone cement material does not require any covering membrane, allowing for a minimally invasive surgery. Flap elevation only requires a full-thickness flap adequate to visualize the bone deficit to be filled. The application of Bond Apatite in ridge augmentation demonstrates very good clinical results and may be considered an alternative to other available graft materials. Additionally, the simple surgical protocol promotes rapid healing in the absence of an inflammatory reaction, and postoperative complications, such as pain or hematomas, are minimized. Treatment costs are also lowered as a result of not having to use a membrane or its fixation devices such as screws or tacks.
Study Overview
Fifty-two cases were treated with a calcium sulphate bone cement (Bond Apatite) according to protocols for thickening narrow ridges in the buccal-lingual dimension. Four or more months (depending on COVID-19 restrictions) following augmentation, implants were placed in the previously grafted sites. Within the study group, six lateral incisors presented with insufficient ridge width following initial graft placement and healing, requiring a second graft placement at the time of implant placement as determined by follow-up cone-beam computed tomography (CBCT). A follow-up prior to the restorative phase of treatment (minimum of 4 months) was conducted of all of the study group cases, and it was noted that all implants in the study group osseointegrated.
The surgical protocol was initiated by prescription of an antibiotic, amoxicillin 500 mg, every 8 hours beginning 1 day before surgery and continuing for 5 days after surgery. Three tablets of dexamethasone, 2 mg, were given 30 minutes before surgery to minimize postoperative swelling and associated pain from inflammation related to flap elevation.
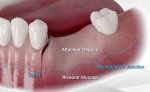
Local anesthesia was administered (articaine 4%, with 1/100,000 epinephrine). A crestal incision was made with a vertical releasing incision that extended 3 mm apical to the mucogingival line so that the releasing incision would be at least 10 mm from the site that would be grafted. An illustration of this type of incision is shown in Figure 1. A full-thickness flap was elevated to adequately visualize the entire defect that was planned for grafting. The flap was extended apical to the mucogingival line to allow flap replacement over the grafted area to attempt incision closure.
Given the biological and physical properties of Bond Apatite, it is not recommended to use a partial-thickness flap. This technique is predicated on not making a periosteal release apically. To maintain integrity of the periosteum, the flap is elongated mesiodistally instead of cutting to enhance coverage. The coronal 3 mm of the flap apical to the mucogingival junction is stretched to achieve more coverage over the already set calcium sulphate. After the flap has been elevated and manipulated, the recipient site can be visualized and prepared. The cortical plate at the defect is now perforated at regular intervals approximately every 5 mm with a surgical bur or piezosurgery tip to connect the host's cells and blood supply to the graft that will be placed at the site. This permits osteogenesis and angiogenesis, facilitating conversion of the graft material to new host bone over the healing period.
Bond Apatite comes in a two-compartment syringe containing the powder (biphasic calcium sulphate and hydroxyapatite crystals) in one compartment and a 9% sodium chloride (NaCl) solution in the second compartment (Figure 2). To activate the material, the shaft of the syringe is advanced forward until the first piston reaches the blue line marked on the syringe tube so that the liquid mixes with the powder to form a wet bone cement. The end cap is removed from the syringe and the material is injected into the osseous defect to be grafted. To allow the material to exit the syringe, the syringe must be tilted about 45 degrees relative to the bone deficit plane. If kept perpendicular to the site surface, the graft material will not extrude from the syringe. It takes about 3 minutes for crystallization to occur and the material to harden. The material needs to be placed into the defect before hardening, otherwise adhesion to the host bone will not occur.
Once the graft material has been deposited in the recipient site, dry sterile gauze is pressed on the surface for 3 to 4 seconds with finger pressure to remove any residual liquid and compress the material to the recipient bone. This is followed by additional compaction of the gauze with a periosteal elevator for 3 to 4 seconds. The material should be well compacted at the site. Once the graft material is mixed the working time before setting occurs is 2 minutes. The surgeon should place the graft material into the site without hesitation to avoid premature setting of the graft. It is not recommended to tap the material into place as this may lead to fracturing of the setting material. The process can be repeated with several successive layers when filling larger/deeper defects that require more than a single syringe.
The flap is then repositioned over the site and a suture is placed at the mesial extent of the crestal incision. This is followed by suturing the distal corner and then the middle part of the incision to fixate the flap in place. Thereafter, suturing is continued by placement of additional sutures as needed to either achieve primary closure or leave a minimal gap of up to 2 mm to 3 mm at the incision line. The vertical releasing incision is then sutured. The authors recommend use of a suture material that lasts 14 to 21 days before it loses its integrity so that adequate tension is present before suture breakdown. Polyglygolic acid, polyglecaprone, or a removable suture like prolene or polytetrafluoroethylene meet these requirements.
With the use of this technique combined with the biocompatibility of the bone cement material, inflammatory issues from the procedure typically are minimal, and patients usually do not require narcotic pain relivers during the initial healing phase. Postoperatively, patients in this study were advised to take acetaminophen, two 500 mg tablets/capsules (1000 mg total dose) every 6 hours if needed for pain.
At 4 months post-surgically, which should allow adequate time for conversion of the graft material, reopening of the site will reveal early host bone with some hydroxyapatite crystals. Histologically, the calcium sulphate portion will have resorbed completely and been replaced with maturing host bone; the larger hydroxyapatite crystals (10% of the original graft material) will have remained, with medium-sized crystals having been resorbed.
In 46 of the 52 patients in the study, implant placement at 4 or more months following lateral augmentation of the planned implant sites was allowed. The remaining six cases had inadequate volume of resulting bone and ridge width to accommodate implant placement and required grafting with additional Bond Apatite and healing prior to implant placement (Table 1).
Study Case Examples
Single Site
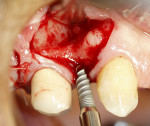
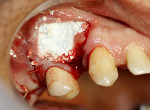
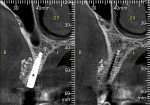
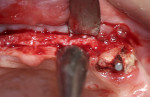
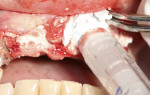
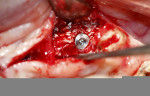
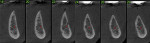
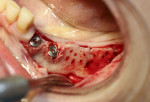
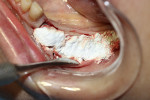
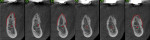
A 58-year-old male patient presented with a desire to replace the missing maxillary right first premolar that had failed to develop in adolescence. A CBCT image was taken to evaluate available bone at the site (Figure 3). The buccal aspect of the ridge presented with a significant deficiency in width to disallow implant placement along the entire implant length; otherwise, a dehiscence would result over the apical half of the implant. The site was flapped and the defect visualized. An osteotomy was made for the implant placement and the implant was inserted (Figure 4). Bond Apatite was mixed in the syringe and injected into the buccal defect and compacted (Figure 5).
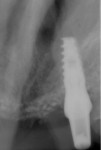
Four months after grafting and immediate implant placement, a CBCT scan demonstrated fill of the buccal defect that had been grafted and the implant encompassed in bone (Figure 6).
Multiple Adjacent Sites
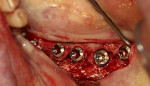
A 66-year-old female patient presented intending to have her full maxillary denture replaced with a fixed implant prosthesis. Examination identified a significant facial defect at the maxillary central and lateral incisors and a knife edge crest that would not permit ridge splitting. This was confirmed upon flap elevation (Figure 7). Bond Apatite was mixed and placed into the facial defect (Figure 8) and compressed, and the flap was closed and secured with sutures.
After 4 months of graft healing, the site was re-entered and ridge width was noted that would allow implant placement (Figure 9). A radiograph was taken following implant placement at the planned site (Figure 10).
Posterior Mandible
A 69-year-old female patient presented with a request for implants to replace the posterior left mandibular teeth that had been missing for several years. A CBCT was taken and cross-sections were analyzed in planning for implant placement. Adequate width was noted mesial to the mental foramen, but inadequate width was revealed distal to the mental foramen (Figure 11). The area was flapped and two implants were placed mesial to the mental foramen. A surgical bur was used to perforate the lateral aspect of the ridge distal to the mental foramen to create bleeding points (Figure 12). Bond Apatite was mixed and placed over the previously perforated bone and over the implants that had just been placed (Figure 13).
Following 4 months of healing to allow the graft to mature and organize, a CBCT was taken. Cross-sectional slices demonstrated a ridge width increase in the area distal to the mental foramen that would allow implant placement (Figure 14). The area was flapped, osteotomies were prepared, and implants were placed (Figure 15).
Discussion
Insufficient ridge width to accommodate implant placement and allow bone to encompass the implant is a common occurrence related to resorption over time following extraction or periodontal disease associated with a tooth that is present or recently extracted. Osseous grafting becomes necessary to modify the remaining ridge to accommodate implant placement. As discussed, various osseous graft materials have been advocated in the literature, all with pros and cons associated with their use. These materials require primary closure of the overlying flap to prevent infection and loss of the graft material; also, placement of a membrane, either resorbable or nonresorbable, is critical to treatment success. With the use of a membrane, treatment costs increase related to the membrane itself as well as fixation devices, such as screws and tacks, necessary to ensure stability of the membrane at the surgical site. Also, the need for a membrane requires additional surgical skill and a longer appointment time to render the treatment. Additionally, more flap release is required to achieve tension-free closure for such procedures.
Ideally, the graft material placed should convert to host bone over time, acting as a spacer and scaffold that allows host cells to create bone within it while stimulating angiogenesis resulting in "live" active bone that can integrate to implants placed within it following healing. Different osseous graft materials have varying rates of resorption. When a bone replacement graft resorbs too quickly, volume is lost as host hard tissue cannot replace the graft fast enough. When resorption is too slow, treatment time is lengthened before implants can be placed. Thus, the material must maintain the required space for a time period that allows the host to replace it at an appropriate rate.
Resorbability is another issue critical to grafting material. Does the graft material fully resorb or do portions remain? How much remains, and does this have a potential to affect integration, decreasing bone-to-implant contact of the implant placed within it? Allografts and autogenous bone partially or fully resorb over a certain period of time. The rate is based on density of the particles, with cortical bone lasting longer then cancellous.27 Xenografts, such as bovine bone, have been reported to not resorb and particles remain encased in connective tissue and host bone.28 Synthetics, depending on their composition, will either fully resorb (calcium sulphate), partially resorb (bioglass), or remain intact long term (hydroxyapatite).29
Implant stability relates to bone-to-implant contact, which can vary depending on numerous factors, such as the implant's micro and macro surface and the type of bone that surrounds the implant after osseous healing and integration. Conversion of the graft material to host bone is critical to long-term maintenance of the bone around the implant. Healing issues around grafted and/or immediate socket implants could lead to inflammation and bone loss.30
Regarding surgical approach, the surgeon cannot always predictably and safely gain full flap release to cover the grafted site due to anatomical or other factors. Risks include severe bleeding and cutting through the mental nerve or other innervating structure at the time of making a periosteal releasing incision.31 In these cases, a few options are available. Ronda and Stacchi discussed an intricate flap design to avoid damaging critical structures during flap elevation.32 They used a "brushing" technique after cutting through the periosteum to help gain release for further coronal coverage. One issue with this technique is that as bleeding increases, it adversely affects the surgeon's ability to see smaller blood vessels or nerves that could be inadvertently cut with a sharp scalpel. Also, by cutting the periosteum, scarring may be induced, which can lead to contraction and shortening of the flap during healing.
During the procedure advocated here, the base of the periosteal tissue is not severed from the rest of the periosteal tissue. Rather, at approximately 3 mm apical to the mucogingival junction, the periosteal tissue is stretched in a coronal direction. In this area, there is minimal muscle so the mucosa will have little tendency to pull back in an apical direction. As Payne and Cobb have shown, if any of the graft material has been left exposed, the body has the ability to epithelialize over it.21 Also, another interesting possible action may occur. If slight tension remains on the periosteal tissue during any part of healing, it may itself induce osteogenesis. Zakaria and coworkers performed a study in 2012 using a specifically designed periosteal distraction process to test this theory.33 Based on other research showing that the inner layer of periosteum is a source of bone cells,34 they showed that constant stretching of this tissue over 6 weeks formed significant bone in a rabbit model.
Soft-tissue and ridge augmentation in humans via a tunnel approach has been well documented. Lee documented 60 human sites of ridge augmentation with follow-up of up to 30 months.35 His technique involves no use of barrier membranes or periosteal flap releasing incisions so as to maintain the integrity of this tissue. Using specially designed instrumentation and a specific combination of graft materials and growth factors, his study showed lateral and vertical augmentation. The results were analyzed using 3-dimensional CBCT scans and histology in addition to clinical documentation for validation of the technique. His procedures are also based on Rapp's assertion that the contribution of native periosteum is null when it is deficient from resultant trauma or other processes.36 As in Lee's technique,35 the process outlined in this article is designed to stretch, not incise, the periosteum and maintain its biologic integrity.
Conclusion
The practitioner is often faced with a situation of a narrow crest, making it difficult or impossible for implant placement and requiring modification of the ridge to allow implants to be utilized. Many factors impact the choice of technique used to expand the ridge laterally. A relatively simple technique that can facilitate ridge width increase while reducing inflammatory and postoperative pain for the patient should be considered. The surgical technique presented here that utilizes Bond Apatite when treating narrow ridges is an innovative, simple, and minimally invasive surgical approach that is related to the material itself. Postoperative pain and inflammation is minimal during healing, which occurs more rapidly due to the conservative surgical approach. This study showed that the material and technique permitted widening of the ridge from 2 mm to up to 6 mm.
Using calcium sulphate-based graft material, such as the one used in this study, has clinical advantages when lateral ridge width requires enhancement to permit implant placement compared to other osseous graft materials. Following healing, 90% of the graft material is converted to host bone over a period of 4 to 6 months depending on the volume of Bone Apatite placed. Thus, bone regeneration, not bone repair, occurs. In addition, use of a collagen membrane and fixation screws/tacks is avoided, helping to reduce the costs of the procedure and making it more affordable for a greater number of patients seeking implant placement in deficient areas of the arches. This method is relatively simple to perform and has fewer potential complications than other ridge augmentation procedures.
DISCLOSURE
The authors had no disclosures to report.
ABOUT THE AUTHORS
David Baranes, DDS
Diplomate, International Congress of Oral Implantologists; Private Practice limited to implantology and bone regeneration, Jerusalem, Israel; international lecturer/teacher specializing in the clinical use of cement for bone regeneration
Gregori M. Kurtzman, DDS;
Former Assistant Clinical Professor, Department of Restorative Dentistry and Endodontics, University of Maryland School of Dentistry; Diplomate, International Congress of Oral Implantologists; Private Practice, Silver Spring, Maryland
Robert A. Horowitz, DDS
Adjunct Clinical Assistant Professor, Departments of Oral and Maxillofacial Surgery and Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York
References
1. Zhao L, Wei Y, Xu T, et al. Changes in alveolar process dimensions following extraction of molars with advanced periodontal disease: a clinical pilot study. Clin Oral Implants Res. 2019;30(4):324-335.
2. Horowitz RA, Levine RA. Bone reconstructive surgery for implant site preparation. Functional Esthetics and Restorative Dentistry. 2007;Series 1, Number 2:20-28.
3. Miron RJ, Hedbom E, Saulacic N, et al. Osteogenic potential of autogenous bone grafts harvested with four different surgical techniques. J Dent Res. 2011;90(12):1428-1433.
4. Becker W, Dahlin C, Becker BE, et al. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: a prospective multicenter study. Int J Oral Maxillofac Implants. 1994;9(1):31-40.
5. Augthun M, Yildirim M, Spiekermann H, Biesterfeld S. Healing of bone defects in combination with immediate implants using the membrane technique. Int J Oral Maxillofac Implants. 1995;10(4):421-428.
6. Lang NP, Hämmerle CH, Brägger U, et al. Guided tissue regeneration in jawbone defects prior to implant placement. Clin Oral Implants Res. 1994;5(2):92-97.
7. Pikos MA. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac Surg Clin North Am. 2005;13(2):91-107.
8. Misch CM, Misch CE. The repair of localized severe ridge defects for implant placement using mandibular bone grafts. Implant Dent. 1995;4(4):261-267.
9. Chaushu G, Mardinger O, Peleg M, et al. Analysis of complications following augmentation with cancellous block allografts. J Periodontol. 2010;81(12):1759-1764.
10. Reininger D, Cobo-Vázquez C, Monteserín-Matesanz M, López-Quiles J. Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(2):e241-e249.
11. Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154-160.
12. Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014;7(suppl 2):S203-S217.
13. Waechter J, Leite FR, Nascimento GG, et al. The split crest technique and dental implants: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2017;46(1):116-128.
14. Sohn DS, Lee HJ, Heo JU, et al. Immediate and delayed lateral ridge expansion technique in the atrophic posterior mandibular ridge. J Oral Maxillofac Surg. 2010;68(9):2283-2290.
15. Toscano N, Holtzclaw D, Mazor Z, et al. Horizontal ridge augmentation utilizing a composite graft of demineralized freeze-dried allograft, mineralized cortical cancellous chips, and a biologically degradable thermoplastic carrier combined with a resorbable membrane: a retrospective evaluation of 73 consecutively treated cases from private practices. J Oral Implantol. 2010;36(6):467-474.
16. Kuperschlag A, Keršytė G, Kurtzman GM, Horowitz RA. Autogenous dentin grafting of osseous defects distal to mandibular second molars after extraction of impacted third molars. Compend Contin Educ Dent. 2020;41(2):76-82.
17. Horowitz RA, Kurtzman GM. Socket preparation for delayed implant placement using a mineralized cancellous allograft. Compend Contin Educ Dent. 2021;42(4):f1-f4.
18. Artzi Z, Weinreb M, Givol N, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004;19(3):357-368.
19. Horowitz RA, Mazor Z, Foitzik C, et al. β-tricalcium phosphate as bone substitute material: properties and clinical applications. J Osseointegration. 2010;2(2):61-68.
20. Sottosanti J, Anson D. Using calcium sulfate as a graft enhancer and membrane barrier. [interview]. Dent Implantol Update. 2003;14(1):1-8.
21. Payne JM, Cobb CM, Rapley JW, et al. Migration of human gingival fibroblasts over guided tissue regeneration barrier materials.J Periodontol. 1996;67(3):236-244.
22. Thomas MV, Puleo DA. Calcium sulfate: properties and clinical applications. J Biomed Mater Res B Appl Biomater. 2009;88(2):597-610.
23. Pecora G, Andreana S, Margarone JE 3rd, et al. Bone regeneration with a calcium sulfate barrier. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(4):424-429.
24. Kim CK, Kim HY, Chai JK, et al. Effect of a calcium sulfate implant with calcium sulfate barrier on periodontal healing in 3-wall intrabony defects in dogs. J Periodontol. 1998;69(9):982-988.
25. Baranes D, Kurtzman GM. Biphasic calcium sulfate as an alternative grafting material in various dental applications. J Oral Implantol. 2019;45(3):247-255.
26. Yahav A, Kurtzman GM, Katzap M, et al. Bone regeneration: properties and clinical applications of biphasic calcium sulfate. Dent Clin North Am. 2020;64(2):453-472.
27. Yang S, Lan L, Miron RJ, et al. Variability in particle degradation of four commonly employed dental bone grafts. Clin Implant Dent Relat Res. 2015;17(5):996-1003.
28. Ohayon L. Histological and histomorphometric evaluation of anorganic bovine bone used for maxillary sinus floor augmentation: a six-month and five-year follow-up of one clinical case. Implant Dent. 2014;23(3):239-244.
29. Doi K, Oue H, Morita K, et al. Development of implant/interconnected porous hydroxyapatite complex as new concept graft material. PLoS One. 2012;7(11):e49051.
30. Bazrafshan N, Darby I. Retrospective success and survival rates of dental implants placed with simultaneous bone augmentation in partially edentulous patients. Clin Oral Implants Res. 2014;25(7):768-773.
31. Greenstein G, Greenstein B, Cavallaro J, et al. Flap advancement: practical techniques to attain tension‐free primary closure. J Periodontol. 2009;80(1):4-15.
32. Ronda M, Stacchi C. A novel approach for the coronal advancement of the buccal flap. Int J Periodontics Restorative Dent. 2015;35(6):795-801.
33. Zakaria O, Madi M, Kasugai S. Induced osteogenesis using a new periosteal distractor. J Oral Maxillofac Surg. 2012;70(3):e225-e234.
34. Takeuchi S, Matsuo A, Chiba H. Beneficial role of periosteum in distraction osteogenesis of mandible: its preservation prevents the external bone resorption. Tohoku J Exp Med. 2010;220(1):67-75.
35. Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): a new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 2017;37(2):165-173.
36. Rapp SJ, Jones DC, Gerety P, Taylor JA. Repairing critical-sized rat calvarial defects with progenitor cell-seeded acellular periosteum: a novel biomimetic scaffold. Surgery. 2012;152(4):595-605.e1.