In Vitro Surface Testing Methods for Dental Implants—Interpretation and Clinical Relevance: A Review
Neel B. Bhatavadekar, BDS, MS, MPH; Amit S. Gharpure, BDS; Nagaraj Balasubramanium, PhD; and E. Todd Scheyer, DDS, MS
Abstract
More than 2,000 dental implant options are estimated to be available for any given clinical situation. Because many implants have claims that are substantiated mainly on the basis of in vitro studies, it is prudent for clinicians to understand the interpretation of such studies and possible clinical relevance. In vitro tests can be segregated as surface analysis tests and mechanical assessment tests. With a wide variation of methodologies used and results achieved by different implant manufacturers, practitioners may find it difficult to judge the clinical significance of in vitro tests. This article provides an overview, including limitations, of the in vitro implant analysis tests implant companies routinely perform, ranging from older methods involving mechanical testing and surface microscopy to more recent tests such as atomic force microscopy (AFM) studies and gene expression tests, to assist clinicians when choosing an implant system. Having identified the limitations of in vitro testing methods, the current evidence indicates that scanning electron microscopy may be useful in providing insight on the role of implant surface topography. AFM, single cell tests, 3D imaging, and gene expression tests could be useful for assessment of cellular and physio-biochemical properties. 3D finite element analysis may help in the evaluation of mechanical properties of dental implants. Clinicians are encouraged to correlate the findings of in vitro tests with robust animal histologic studies and well-designed, high-quality clinical research to ascertain optimum clinical results.
Over the past few decades, rapid technological development has advanced implant dentistry. Today, new implant designs and surfaces are being tested and manufactured, and it is estimated that more than 2,000 implant options are available for a given clinical scenario.1 With a wide array of choices available to clinicians, evidence-based selection of an optimum implant for a given situation can become difficult. Previous studies have looked at evidence from well-established publications to provide a suitable guide to aid clinicians in making educated choices regarding implant selection.1,2 However, little is discussed in the literature about the importance of in vitro study designs and the role they play in assessing clinical implant success.
Most of the claims dental implant companies make rely primarily on in vitro studies.2,3 Thus, an obvious need exists for a comprehensive overview of available in vitro study designs. Current studies on in vitro testing are limited and vary in their materials, methodologies, and quality. Any attempt at conducting a systematic review would have inevitable shortcomings. Hence, the aim of this article is to provide a literature overview of the current data of in vitro testing and assist clinicians in understanding the clinical relevance of such testing.
Many currently available dental implants have complex designs, and this poses several challenges when assessing topographical and analytical surface characterizations. However, an ongoing need for in-depth surface characterization assessment exists because implant surfaces have been shown to influence the primary interfacial reactions with bone; epithelial and connective tissue cells,4-6 such as macromolecular adsorption/desorption and conformational changes; as well as cell adhesion, proliferation, and differentiation.7 Additionally, the potential role of surface roughness and texture in influencing the early wound healing processes at the blood-implant interface has also been identified.8 Besides the surface characteristics of implants, the geometry and macro-design also play an integral role in osseointegration. Various mechanical factors create an effective implant-bone interface. Implant macro-design and geometric features determine the primary stability and stress distribution during osseointegration and the capacity of implants to withstand forces during the process of osseointegration.9 Therefore, the optimal implant design and thread geometry, including shape, pitch, width and depth, and crestal module, can potentially affect the osseointegration process and the primary and secondary stability of the implant.10
Although implant design has seen significant advancement, the standard of in vitro testing has remained relatively unchanged with limited technological improvements. Also, standardization protocols for testing across studies and laboratories are lacking, thereby making comparisons extremely difficult. Clinicians often choose an implant for a given surgical situation based on the product claims put forth by the manufacturer. Such claims, however, may not be supported by long-term human trials, but rather by in vitro tests, and potentially could be incorrectly extrapolated to a clinical situation.1,2 Hence, it is imperative that clinicians understand the existing in vitro tests, including their limitations, utilized by implant companies to be better equipped to make clinical choices.
This article presents an overview of the advantages and disadvantages of in vitro techniques currently used for implant testing, with the intention of helping clinicians better assess how such testing may or may not relate to the clinical claims made by the manufacturer. To the best of the authors' knowledge, this is the first review exploring this association in considerable detail.
Classification of Implant Testing Designs
Dental implant testing can be broadly divided into in vitro and in vivo study designs. Various in vitro testing methods are identified in Table 1. In vitro testing can be generally classified into two types:
1. Surface testing includes: (a) assessment of physical characteristics and topographic properties, such as surface energy, charge, surface roughness, and irregularities; and (b) assessment of physio-biochemical properties (cell-substrate interface), such as ability of cells to adhere to, spread, and differentiate.
2. Mechanical testing primarily comprises: static testing, torque testing, failure evaluation, tension testing, fatigue testing, stress pattern, and life cycle using mechanical testing machines, and finite element analysis (FEA).
Topographic and Physical Surface Testing
Properties of an implant surface play an important role in the success and biocompatibility of the implant.11,12 The effects of various factors such as surface energy, composition, roughness, and topography are integral to osseointegration, during both initial and late phases of biologic healing.12
Among the various tests to measure surface characteristics, surface electron microscopies for topographic analysis have been the most commonly used. Methods to assess implant topography include non-contact laser profilometry, interference microscopy, stereo-scanning electron microscopy (stereo-SEM), and atomic force microscopy (AFM) (Table 1).13 Further variations of microscopy such as focused ion beam microscopy and high-resolution transmission electron microscopy also have been used to provide microstructural and chemical data.14 Although the effectiveness of wavelength-dependent roughness approaches to describe surface topographies has been demonstrated in previous studies,13 a strong emphasis has been placed on the importance of using 3-dimensional (3D) analysis to provide information about amplitude as well as spatial or hybrid data.15,16 Amplitude is surface height and is measured as the differences between the highest peaks and deepest valleys. Spatial parameters describe the texture of the surface and the horizontal distance between irregularities such as randomness and periodicity. Hybrid data is a combination of spatial and amplitude characteristics and is affected by changes in either.17
Other techniques such as stylus profilometry,18,19 optical scanning methods like interferometry,20-22 or confocal laser scanning microscopy,15,18,23 have been applied for quantitative assessment of 3D implant surface roughness. However, different measurement techniques have reported different roughness values.14 Hence, there is significant disparity in the validity of the data on surface roughness. The literature has stressed the importance of using appropriate measurement filters for tops, valleys, and flanks of the implant surface,15,16 and the use of different filters may be the cause of such variable data. Furthermore, the absence of unfiltered raw data relating to the scanned topographies also might be an issue preventing the comparison of published results.24
Recently, there has been interest in the use of x-ray photoelectron spectroscopy for surface element analysis and chemical composition.25 On comparison with SEM, however, variations in this method's ability to discriminate between different topographies have caused researchers to reconsider current approaches for the topographical evaluation of implant surfaces.24 Unfortunately, the standards of surface metrology used in published articles show high variability and inconsistent quality, and previous attempts at a systematic review on the impact of surface roughness in bone healing have demonstrated unacceptable standards for reporting surface metrology.26 Further, variabilities in terminology among different articles, wherein terms like "roughness" and/or "smoothness" are not adequately defined, present difficulties in drawing conclusions.16 Therefore, further refinement in experimental settings and terminologies needs to be considered for future studies on this subject.
Within the limitations of the current evidence, optical methods may be acceptable tools to analyze surface roughness because they are fast and have wide measuring ranges, in both vertical and lateral directions.15 Additionally, SEM may be well-suited for topographical evaluation, as this medium provides a large depth of field with high spatial resolution down to the nanometer range, appropriate for studying structures with a high aspect ratio.13 The nature of bone biology and mechanisms of osseointegration, however, are complex and are affected by multiple factors besides implant surface topography. Although results of SEM studies may be more useful than other currently available in vitro topographic tests, their results need to be verified by other in vitro, in vivo, and clinical tests. Although SEM has certain shortcomings, it may provide limited insight on the role of surface topography on clinical results.
Physio-Biochemical Surface Testing
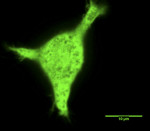
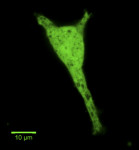
The behavior of cells on the implant is vital to its success. Osteoblasts constitute the primary cell type that drives osteointegration. Several studies looked at the effect implant surface roughness has on osteoblast proliferation, differentiation, and protein synthesis whereby human osteoblast-like cells (MG63) were cultured on titanium discs that had been prepared by different treatment regimens.27-29 These results demonstrate that surface roughness alters osteoblast proliferation, differentiation, and matrix production in vitro. The results also suggest that implant surface roughness may play a role in determining phenotypic expression of cells in vivo. Peri-implant soft tissues also play a significant role when implant length is decreased (8 mm or less), as they may encompass more than one-third of the height area. Fibrous encapsulation involving the soft tissue requires the response of fibroblasts, epithelial cells, and keratinocytes, which have distinct growth patterns and adhesion capabilities to the implant.30 Adherence of gingival fibroblasts and oral keratinocytes to implant surfaces also has been evaluated in this context by SEM analysis.31 The effect of changing grain size also has been tested.32 Implants have previously been coated with matrix proteins (such as collagen and sulfonated hyaluronan) to support cell binding.33 Such cellular behavior in context of the implant has been studied in a 2-dimensional (2D) microenvironment, while cellular behavior can now be imaged and visualized in 3D microenvironments (Figure 1 through Figure 6).
Previous studies relating to cell adhesion and response on implant surfaces primarily involved cell attachment and washout, and subsequently used SEM to measure the number of cells remaining on the surface after washing.34-37 This yielded a preliminary assessment of adhesion strength but did reveal how the cell might behave with the implant surface. Also, because the washout tests relied on the presence or absence of cells after washout, they did not provide quantitative data at the single cell level, which can be crucial since controlling for confounding factors can be easier at the single cell level. In the past, centrifugation was used to determine the average force needed to remove cells from a solid support.38 These studies measured an average detachment force but were unable to localize and measure the force needed to detach a single cell.
Cytodetachment is another method of physio-biochemical surface testing that involves examining single cell responses to implant material surfaces. This method promises a significant improvement over conventional technique in studying cell interactions with implant surfaces.39 With cytodetachment technology, osteoblasts are allowed to attach to a surface, and a nanoprobe is positioned adjacent to the cell. The probe is then moved using a piezo actuator to either nudge the cell or completely detach it (Figure 7 and Figure 8). The displacement applied to the piezo actuator in comparison to the actual observed displacement provides an indication of how much the cell is resisting, and, thus, the cell detachment force can be calculated. Various models can then be developed for both the nudging or the complete detachment, and to provide quantitative parameters that describe cell mechanics and, in turn, the cellular response to an implant surface.39
In addition to detachment tests, there has been a strong focus on determining gene expression of cells on implant surfaces.25,27 These studies aim to review differential expression of genes that could support matrix production and growth of osteoblasts (promoting osseointegration) or fibroblasts and epithelial cells (promoting soft-tissue culture). Various tests have been targeted to study gene expression, such as regulation of mRNA, DNA quantification, and protein quantification. The response of macrophages to implant surfaces can be demonstrated by their expression and release of anti-inflammatory markers and their immunomodulatory effect toward anti-inflammatory macrophage activation.25 The effect of improved regulation of the inflammatory components may provide some insight into the amount of osseointegration and reduction in clinical healing time. Thus, among the various biochemical testing methods currently available, gene expression and cytodetachment tests seem to provide the most valuable and clinically relevant results in terms of cell behavior on implant surfaces. The findings of these tests, however, need to be confirmed and correlated with in vivo animal and clinical studies.
Mechanical Testing
Most commonly used mechanical tests involve systems that utilize static testing, torque testing, failure evaluation, tension testing, fatigue testing, stress pattern, and life cycle.40,41 According to ISO 14801:2016, Dentistry - Implants - Dynamic loading test for endosseous dental implants, dental implant developers must have an accredited laboratory perform fatigue testing to compare endosseous dental implants of different designs and sizes. Each implant size and shape must be tested to pass regulatory approval, necessitating that the manufacturer have a testing partner who understands the unique requirements of dental implant testing. This ISO standard is an update to ISO 14081:2007, a previous version that did not explicitly outline static testing design. The new version contains significant changes that address the shortcomings of the 2007 report and describes fatigue tests for implants in detail. ISO 14801:2016 is an integral safeguard for maintaining the quality of implants used in clinical practice. In an effort to achieve the best possible clinical results, clinicians are advised to check with implant companies to ensure they are in conformance with the latest ISO standards.
Mechanical tests based on FEA have also been reported in the literature for testing implant fixtures as well as prosthetic components.42-44 FEA has been used extensively for prediction of biomechanical performance of dental implant systems.45 This method is based on the principle of dividing a single complex mechanical problem into smaller and simpler domains in which the individual variables can be interpolated by using shape functions. FEA can be 2D or 3D. Although 2D FEA may be useful in analyzing mechanical behavior of a single-tooth unit, its quantitative stress analysis is less reliable than 3D because it overestimates its results and may not adequately represent complex anatomical configurations of dental structures.46,47
It should be noted that FEA is based on computer models, and although boundary limits may be set, this method broadly tends to consider all dental tissues to be isotropic and homogenous.45 Thus, until advanced digital imaging techniques can successfully be used to model bone geometry in greater detail for accurate representation of structurally complex non-homogenous implant-bone structures, FEA results should be interpreted with caution.
Clinical Relevance
Dental implant companies generally face tremendous competitive pressure and produce a list of claims for their implant systems that supports their conclusion of clinical superiority over a competitor's system. In a previous article, the author (NBB) assessed if such claims could be substantiated with the evidence available.3 Many of them could not be substantiated by well-controlled randomized clinical trials, and many claims relied only on in vitro data.3 A unified European certification board (CE) and the US Food and Drug Administration regulate the accreditation of endosseous dental implants. Submission of a premarket notification (510[k]), consisting of documentation that the new implant has substantial equivalence to a product that is already on the market, is one way to get an implant accredited.3 In this scenario, the implant company may not need to perform rigorous randomized clinical trials. Therefore, it is critical for clinicians to interpret the existing data regarding in vitro testing before extrapolating it directly to a clinical scenario.
The authors assessed the available literature on implant testing (search words: dental implants, titanium implants, osseointegration, in vitro, testing, laboratory techniques and procedures, surface properties, surface roughness parameters, 3D-SEM, AFM, surface chemistry, surface micro roughness, surface nanoroughness, optical interferometry, surface physics, osteoblast, cell attachment, cytokine, gene expression, inflammatory response, macrophage) and found that there was substantial variation among the types and methods of in vitro tests. A lack of standardization makes it difficult to extrapolate in vitro testing results to the clinical setting. Judging by the current evidence, optical methods seem to be useful in assessing surface irregularities. SEM may be beneficial in evaluating topographic details of implant surfaces. Newly developed 3D FEA models could provide a more comprehensive picture of mechanical properties of implants than current mechanical testing methods. Further advances in biological testing, such as cytodetachment and gene expression studies like regulation of mRNA, DNA quantification, and protein quantification, may provide insight into the biologic response of tissues to the implant surfaces.
Different in vitro methods provide perspective on different aspects of implant designs. Clinicians should review all three methodologies-physical, mechanical, and biologic testing results-to gain a better undertaking of implant designs. Future efforts should be directed toward standardizing in vitro tests for comparison and for effective clinical extrapolation of results, and attaining stringent regulation from governing bodies before commercial distribution. Research plans could further incorporate these in vitro testing methods (eg, cytodetachment) with other tests (eg, pull-out tests) to see if a clinical correlation exists.
Conclusion
A combination of various testing methods needs to be utilized to provide a comprehensive overview of the physical, mechanical, chemical, and biological characteristics of a dental implant. Before selecting an implant for clinical use, clinicians should ensure that it conforms to current ISO 14801:2016 standards for mechanical testing. Currently, results from FEA and SEM provide the best assessment of physical and surface properties, and cytodetachment and gene expression tests offer the most accurate assessments of physio-biochemical properties. It is prudent for clinicians to utilize multiple parameters with the understanding that each in vitro test has its own share of limitations. Although this review was an attempt to provide clarity on the various in vitro testing methods and present findings in a reliable fashion, the authors strongly recommend that clinicians correlate these findings with animal models and high-quality clinical studies.
Acknowledgment
The authors thank Ms. Trupti Thite for processing and recording of laboratory images.
About the Authors
Neel B. Bhatavadekar, BDS, MS, MPH
Private Practice, Pune, India
Amit S. Gharpure, BDS
Graduate Student,Department of Periodontics, School of Dentistry, University of Washington, Seattle, Washington
Nagaraj Balasubramanium, PhD
Associate Professor, Indian Institute of Science Education and Research, Pune, India
E. Todd Scheyer, DDS, MS
Clinical Assistant Professor, University of Texas Dental Branch, Houston, Texas; Private Practice, Houston, Texas
References
1. Jokstad A, Braegger U, Brunski JB, et al. Quality of dental implants. Int Dent J. 2003;53(6 suppl 2):409-443.
2. Bhatavadekar N. Helping the clinician make evidence-based implant selections. A systematic review and qualitative analysis of dental implant studies over a 20 year period. Int Dent J. 2010;60(5):359-369.
3. Bhatavadekar N. Clinical decisions and the quality of evidence available for dental implants. J Periodontol. 2009;80(10):1559-1561.
4. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(suppl 4):172-184.
5. Yamano S, Al-Sowygh ZH, Gallucci GO, et al. Early peri-implant tissue reactions on different titanium surface topographies. Clin Oral Implants Res. 2011;22(8):815-819.
6. Rompen E, Domken O, Degidi M, et al. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006;17(suppl 2):55-67.
7. Wennerberg A, Svanborg LM, Berner S, Andersson M. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implants Res. 2013;24(2):203-209.
8. Gittens RA, Scheideler L, Rupp F, et al. A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater. 2014;10(7):2907-2918.
9. Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2000. 2008;47:51-66.
10. Ryu HS, Namgung C, Lee J-H, Lim YJ. The influence of thread geometry on implant osseointegration under immediate loading: a literature review. J Adv Prosthodont. 2014;6(6):547-554.
11. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(2):155-170.
12. Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7(4):329-345.
13. Wieland M, Textor M, Spencer ND, Brunette DM. Wavelength-dependent roughness: a quantitative approach to characterizing the topography of rough titanium surfaces. Int J Oral Maxillofac Implants. 2001;16(2):163-181.
14. Jarmar T, Palmquist A, Brånemark R, et al. Characterization of the surface properties of commercially available dental implants using scanning electron microscopy, focused ion beam, and high-resolution transmission electron microscopy. Clin Implant Dent Relat Res. 2008;10(1):11-22.
15. Wennerberg A, Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 2000;15(3):331-344.
16. Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25(1):63-74.
17. Dong WP, Sullivan PJ, Stout KJ. Comprehensive study of parameters for characterising three-dimensional surface topography: IV: parameters for characterising spatial and hybrid properties. Wear. 1994;178(1-2):45-60.
18. Al-Nawas B, Grotz KA, Götz H, et al. Validation of three-dimensional surface characterising methods: scanning electron microscopy and confocal laser scanning microscopy. Scanning. 2001;23(4):227-231.
19. Rupp F, Scheideler L, Rehbein D, et al. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004;25(7-8):1429-1438.
20. Arvidsson A, Sater BA, Wennerberg A. The role of functional parameters for topographical characterization of bone-anchored implants. Clin Implant Dent Relat Res. 2006;8(2):70-76.
21. Sul YT, Byon E, Wennerberg A. Surface characteristics of electrochemically oxidized implants and acid-etched implants: surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int J Oral Maxillofac Implants. 2008;23(4):631-640.
22. Valverde GB, Jimbo R, Teixeira HS, et al. Evaluation of surface roughness as a function of multiple blasting processing variables. Clin Oral Implants Res. 2013;24(2):238-242.
23. Al-Nawas B, Götz H. Three-dimensional topographic and metrologic evaluation of dental implants by confocal laser scanning microscopy. Clin Implant Dent Relat Res. 2003;5(3):176-183.
24. Kournetas N, Spintzyk S, Schweizer E, et al. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent Mater. 2017;33(8):e317-e327.
25. Hotchkiss KM, Ayad NB, Hyzy SL, et al. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin Oral Implants Res. 2017;28(4):414-423.
26. Shalabi MM, Gortemaker A, Van't Hof MA, et al. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85(6):496-500.
27. Martin JY, Schwartz Z, Hummert TW, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29(3):389-401.
28. Bowers KT, Keller JC, Randolph BA, et al. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992;7(3):302-310.
29. Schwartz Z, Martin JY, Dean DD, et al. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996;30(2):145-155.
30. Teng FY, Ko CL, Kuo HN, et al. A comparison of epithelial cells, fibroblasts, and osteoblasts in dental implant titanium topographies. Bioinorg Chem Appl. 2012;2012:687291.
31. Dorkhan M, Yücel-Lindberg T, Hall J, et al. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health. 2014;14:75.
32. Babuska V, Dobra J, Kulda V, et al. Comparison of fibroblast and osteoblast response to cultivation on titanium implants with different grain sizes. J Nanomater. 2015;2015:1-9. https://doi.org/10.1155/2015/920893.
33. Schulz MC, Korn P, Stadlinger B, et al. Coating with artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants. J Mater Sci Mater Med. 2014;25(1):247-258.
34. Cooper LF, Zhou Y, Takebe J, et al. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27(6):926-936.
35. Isa ZM, Schneider GB, Zaharias R, et al. Effects of fluoride-modified titanium surfaces on osteoblast proliferation and gene expression. Int J Oral Maxillofac Implants. 2006;21(2):203-211.
36. Marinucci L, Balloni S, Becchetti E, et al. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro. Int J Oral Maxillofac Implants. 2006;21(5):719-725.
37. Lumbikanonda N, Sammons R. Bone cell attachment to dental implants of different surface characteristics. Int J Oral Maxillofac Implants. 2001;16(5):627-636.
38. Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109(4 Pt 1):1795-1805.
39. Bhatavadekar N, Hu J, Keys K, et al. Novel application of cytodetachment technology to the analysis of dental implant surfaces. Int J Oral Maxillofac Implants. 2011;26(5):985-990.
40. Rangert B, Gunne J, Sullivan DY. Mechanical aspects of a Brånemark implant connected to a natural tooth: an in vitro study. Int J Oral Maxillofac Implants. 1991;6(2):177-186.
41. Möllersten L, Lockowandt P, Lindén LA. Comparison of strength and failure mode of seven implant systems: an in vitro test. J Prosthet Dent. 1997;78(6):582-591.
42. Sakaguchi RL, Borgersen SE. Nonlinear finite element contact analysis of dental implant components. Int J Oral Maxillofac Implants. 1993;8(6):655-661.
43. van Rossen IP, Braak LH, de Putter C, de Groot K. Stress-absorbing elements in dental implants. J Prosthet Dent. 1990;64(2):198-205.
44. Haack JE, Sakaguchi RL, Sun T, Coffey JP. Elongation and preload stress in dental implant abutment screws. Int J Oral Maxillofac Implants. 1995;10(5):529-536.
45. Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. J Prosthet Dent. 2001;85(6):585-598.
46. Romeed SA, Fok SL, Wilson NH. A comparison of 2D and 3D finite element analysis of a restored tooth. J Oral Rehabil. 2006;33(3):209-215.
47. Poiate IA, Vasconcellos AB, Mori M, Poiate E Jr. 2D and 3D finite element analysis of central incisor generated by computerized tomography. Comput Methods Programs Biomed. 2011;104(2):292-299.
Associate Professor, Indian Institute of Science Education and Research, Pune, India
E. Todd Scheyer, DDS, MS
Clinical Assistant Professor, University of Texas Dental Branch, Houston, Texas; Private Practice, Houston, Texas
References
1. Jokstad A, Braegger U, Brunski JB, et al. Quality of dental implants. Int Dent J. 2003;53(6 suppl 2):409-443.
2. Bhatavadekar N. Helping the clinician make evidence-based implant selections. A systematic review and qualitative analysis of dental implant studies over a 20 year period. Int Dent J. 2010;60(5):359-369.
3. Bhatavadekar N. Clinical decisions and the quality of evidence available for dental implants. J Periodontol. 2009;80(10):1559-1561.
4. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(suppl 4):172-184.
5. Yamano S, Al-Sowygh ZH, Gallucci GO, et al. Early peri-implant tissue reactions on different titanium surface topographies. Clin Oral Implants Res. 2011;22(8):815-819.
6. Rompen E, Domken O, Degidi M, et al. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006;17(suppl 2):55-67.
7. Wennerberg A, Svanborg LM, Berner S, Andersson M. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implants Res. 2013;24(2):203-209.
8. Gittens RA, Scheideler L, Rupp F, et al. A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater. 2014;10(7):2907-2918.
9. Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2000. 2008;47:51-66.
10. Ryu HS, Namgung C, Lee J-H, Lim YJ. The influence of thread geometry on implant osseointegration under immediate loading: a literature review. J Adv Prosthodont. 2014;6(6):547-554.
11. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(2):155-170.
12. Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7(4):329-345.
13. Wieland M, Textor M, Spencer ND, Brunette DM. Wavelength-dependent roughness: a quantitative approach to characterizing the topography of rough titanium surfaces. Int J Oral Maxillofac Implants. 2001;16(2):163-181.
14. Jarmar T, Palmquist A, Brånemark R, et al. Characterization of the surface properties of commercially available dental implants using scanning electron microscopy, focused ion beam, and high-resolution transmission electron microscopy. Clin Implant Dent Relat Res. 2008;10(1):11-22.
15. Wennerberg A, Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 2000;15(3):331-344.
16. Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25(1):63-74.
17. Dong WP, Sullivan PJ, Stout KJ. Comprehensive study of parameters for characterising three-dimensional surface topography: IV: parameters for characterising spatial and hybrid properties. Wear. 1994;178(1-2):45-60.
18. Al-Nawas B, Grotz KA, Götz H, et al. Validation of three-dimensional surface characterising methods: scanning electron microscopy and confocal laser scanning microscopy. Scanning. 2001;23(4):227-231.
19. Rupp F, Scheideler L, Rehbein D, et al. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004;25(7-8):1429-1438.
20. Arvidsson A, Sater BA, Wennerberg A. The role of functional parameters for topographical characterization of bone-anchored implants. Clin Implant Dent Relat Res. 2006;8(2):70-76.
21. Sul YT, Byon E, Wennerberg A. Surface characteristics of electrochemically oxidized implants and acid-etched implants: surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int J Oral Maxillofac Implants. 2008;23(4):631-640.
22. Valverde GB, Jimbo R, Teixeira HS, et al. Evaluation of surface roughness as a function of multiple blasting processing variables. Clin Oral Implants Res. 2013;24(2):238-242.
23. Al-Nawas B, Götz H. Three-dimensional topographic and metrologic evaluation of dental implants by confocal laser scanning microscopy. Clin Implant Dent Relat Res. 2003;5(3):176-183.
24. Kournetas N, Spintzyk S, Schweizer E, et al. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent Mater. 2017;33(8):e317-e327.
25. Hotchkiss KM, Ayad NB, Hyzy SL, et al. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin Oral Implants Res. 2017;28(4):414-423.
26. Shalabi MM, Gortemaker A, Van't Hof MA, et al. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85(6):496-500.
27. Martin JY, Schwartz Z, Hummert TW, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29(3):389-401.
28. Bowers KT, Keller JC, Randolph BA, et al. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992;7(3):302-310.
29. Schwartz Z, Martin JY, Dean DD, et al. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996;30(2):145-155.
30. Teng FY, Ko CL, Kuo HN, et al. A comparison of epithelial cells, fibroblasts, and osteoblasts in dental implant titanium topographies. Bioinorg Chem Appl. 2012;2012:687291.
31. Dorkhan M, Yücel-Lindberg T, Hall J, et al. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health. 2014;14:75.
32. Babuska V, Dobra J, Kulda V, et al. Comparison of fibroblast and osteoblast response to cultivation on titanium implants with different grain sizes. J Nanomater. 2015;2015:1-9. https://doi.org/10.1155/2015/920893.
33. Schulz MC, Korn P, Stadlinger B, et al. Coating with artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants. J Mater Sci Mater Med. 2014;25(1):247-258.
34. Cooper LF, Zhou Y, Takebe J, et al. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27(6):926-936.
35. Isa ZM, Schneider GB, Zaharias R, et al. Effects of fluoride-modified titanium surfaces on osteoblast proliferation and gene expression. Int J Oral Maxillofac Implants. 2006;21(2):203-211.
36. Marinucci L, Balloni S, Becchetti E, et al. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro. Int J Oral Maxillofac Implants. 2006;21(5):719-725.
37. Lumbikanonda N, Sammons R. Bone cell attachment to dental implants of different surface characteristics. Int J Oral Maxillofac Implants. 2001;16(5):627-636.
38. Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109(4 Pt 1):1795-1805.
39. Bhatavadekar N, Hu J, Keys K, et al. Novel application of cytodetachment technology to the analysis of dental implant surfaces. Int J Oral Maxillofac Implants. 2011;26(5):985-990.
40. Rangert B, Gunne J, Sullivan DY. Mechanical aspects of a Brånemark implant connected to a natural tooth: an in vitro study. Int J Oral Maxillofac Implants. 1991;6(2):177-186.
41. Möllersten L, Lockowandt P, Lindén LA. Comparison of strength and failure mode of seven implant systems: an in vitro test. J Prosthet Dent. 1997;78(6):582-591.
42. Sakaguchi RL, Borgersen SE. Nonlinear finite element contact analysis of dental implant components. Int J Oral Maxillofac Implants. 1993;8(6):655-661.
43. van Rossen IP, Braak LH, de Putter C, de Groot K. Stress-absorbing elements in dental implants. J Prosthet Dent. 1990;64(2):198-205.
44. Haack JE, Sakaguchi RL, Sun T, Coffey JP. Elongation and preload stress in dental implant abutment screws. Int J Oral Maxillofac Implants. 1995;10(5):529-536.
45. Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. J Prosthet Dent. 2001;85(6):585-598.
46. Romeed SA, Fok SL, Wilson NH. A comparison of 2D and 3D finite element analysis of a restored tooth. J Oral Rehabil. 2006;33(3):209-215.
47. Poiate IA, Vasconcellos AB, Mori M, Poiate E Jr. 2D and 3D finite element analysis of central incisor generated by computerized tomography. Comput Methods Programs Biomed. 2011;104(2):292-299.