Contemporary Use of Bioactive Materials in Restorative Dentistry
Gerard Kugel, DMD, MS, PhD; and Steven Eisen, DMD
Abstract: While not a new concept in dentistry, bioactive restorative materials continue to show further potential, as new products appear to offer significant benefits for both clinicians and patients. Such materials can form a surface layer of an apatite-like substance in the presence of an inorganic phosphate solution. Their applications in dentistry include remineralization of dentin, maintenance of long-term bonded restorations, and repair of intrabony defects. This article reviews new developments in this expanding area of dentistry and highlights the still untapped promise that bioactive materials hold for practitioners.
The concept of a bioactive material was first recognized in 1969. Early on, bioactivity was defined as follows: “A bioactive material is one that elicits a specific biological response at the interface of the material which results in the formation of a bond between the tissues and the material.”1 Since then, the area of bioactive materials has expanded enormously in both medicine and dentistry.
Hench introduced the original criteria for the evaluation of bioactivity of a material. In 1994 he proposed a classification2 in which he divided bioactive materials into two groups: osteoproductive and osteoconductive. In osteoproductive materials, the bioactive surface is colonized by osteogenic stem cells. Bioactivity occurs when a material elicits both an intracellular and extracellular response at its interface (eg, bioglass materials are both osteoproductive and osteoconductive). Osteoconductive materials provide a biocompatible interface, along which bone migrates. Osteoconductive bioactivity occurs when a material elicits only an extracellular response at its interface (eg, synthetic hydroxyapatite [HA]).
The idea of bioactive restorative dental materials is not new. When one considers the concept of adhesion to tooth structure along with the release of fluoride, and more recently calcium and phosphate to help prevent recurrent decay, it is clear that “bioactive” restorative materials have been available in dentistry for many years in the form of fluoride-releasing materials. It now seems well accepted that a bioactive material is defined as one that forms a surface layer of an apatite-like material in the presence of an inorganic phosphate solution.3 Materials that are only fluoride-releasing such as resin-modified glass ionomers (RMGIs) are not truly bioactive and do not fall under either the osteoproductive or osteoconductive groups. Glass ionomers release fluoride ions in an effort to prevent secondary caries; however, the development of caries is still a major reason for clinical failure of glass-ionomer–cemented restorations.4 Glass ionomers are excellent materials for many situations and clinical indications, but their ability to prevent recurrent caries may be somewhat questionable and they do not form HA.
Integration Into Dentistry
Many dentists are already integrating bioactive materials into their practices, as calcium hydroxide and mineral trioxide aggregate (MTA) have been available for a long time. One of the earliest uses of bioactive materials in dentistry involved calcium hydroxide, which clinicians have used for decades. It dissociates into calcium and hydroxyl ions, and these calcium ions reduce capillary permeability, lessen the serum flow, and decrease the levels of inhibitory pyrophosphates that cause mineralization. The hydroxyl ions neutralize acid produced by osteoclasts, maintaining optimum pH levels for pyrophosphatase activity, leading to an increased level of calcium-dependent pyrophosphatase—this reduces the levels of inhibitory pyrophosphates and causes mineralization. This activity makes calcium hydroxide one of dentistry’s first bioactive materials, and it remains one of the most widely used.
Some new bioactive filling materials are based on glass-ionomer chemistry. One advantage of these new materials is their ability to inhibit surface matrix metalloproteinases (MMPs). The plasma proteins released by dentin when subjected to acids from caries will cause hydrolytic and enzymatic (MMP) breakdown of the dentin and resin bonding-agent interface.4,5 Several methods for reducing these MMPs include the use of 2% chlorhexidine, etchants containing benzalkonium chloride, and polyvinylphosphonic acid-producing products such as the new RMGI-based bioactive restoratives.
An example of a long-standing and well-accepted bioactive material in dentistry is mineral trioxide aggregate (MTA). It is a mechanical mixture of three powder ingredients—Portland cement (75%), bismuth oxide (20%), and gypsum (5%)6—along with trace amounts of silicon dioxide, calcium oxide, magnesium oxide, potassium sulfate, and sodium sulfate. The major component, Portland cement, is a mixture of tricalcium aluminate, dicalcium silicate, tricalcium silicate, and tetracalcium aluminoferrite. Sarkar et al7 concluded that MTA is not an inert material because it dissolves, releasing all of its major cationic components and triggering the precipitation of HA on its surface and in the surrounding fluid. It appears to bond chemically to dentin when placed against it, possibly by a diffusion reaction between its apatitic surface and dentin. MTA has a long history of clinical success and, in terms of its biocompatibility, an ability to seal and produce dentinogenic activity.
MTA was recently introduced in a user-friendly, light-activated form (ie, TheraCal, Bisco, Inc., www.bisco.com). This light-cured bioactive material is used to seal and protect the dentin–pulp complex and can be used for pulp capping. Some have referred to this new class of internal pulpal protectant materials as resin-modified calcium silicate (RMCS). While RMCS has demonstrated apatite formation,7 it has yet to be determined if this new class of materials is clinically effective.
Another entry into the bioactive arena was developed as a multipurpose dentin and root replacement material (ie, Biodentine®, Septodont, www.septodontusa.com). It has clinical indications that go beyond those of MTA and related Portland cement/calcium-silicate products. These include restoration of deep and large coronal carious lesions and deep cervical and radicular lesions, as well as MTA indications such as pulp capping and pulpotomy, repair of root perforations, furcation perforations, perforating internal resorptions, external resorption, apexification, and root-end filling in endodontic surgery, and it is biocompatible.8 The product is also bioactive with deposition of HA on its cement surface in the presence of simulated body fluid.9
Recent developments have also occurred in the area of bioactive cements. These include calcium aluminates (ie, Ceramir®, Doxa Dental Inc., www.ceramirus.com) and calcium-, phosphate-, and fluoride-releasing cements. These cements produce HA at 14 and 28 days (Figure 1 and Figure 2).10 A bioactive material that has the ability to induce HA formation on a damaged tooth structure while meeting clinical standards may have beneficial clinical implications. One hypothesis is that the use of such a material, whether as a restorative product or cement, would make it more difficult for recurrent caries to occur, because the natural formation of HA between the tooth structure and the material should create a more stable cement interface. These benefits also hold for this same class of materials that is being used as liners and restoratives.11
These cements are intended for the permanent cementation of crowns and fixed partial dentures, gold inlays and onlays, prefabricated metal cast dowels and cores, and high-strength all-zirconia restorations.11,12 The calcium aluminate cement (Ceramir) is a water-based composition comprising calcium aluminate and glass-ionomer components.13
New calcium-, phosphate-, and fluoride-releasing bioactive cements (eg, Activa™ Bioactive, Pulpdent, www.activabioactive.com; BioCem®, NuSmile, www.nusmilecrowns.com) contain a glass-ionomer component.14 This contributes to their initial, short-duration pH reading; improved flow and setting characteristics; early adhesive properties to tooth structure; and early strength properties. The components in these cements seem to result in increased strength and retention over time; biocompatibility; sealing of tooth–material interface; bioactivity–apatite formation; stable, sustained long-term properties; lack of solubility/degradation; and ultimate development of a stable, basic cement pH measurement.12
To reiterate, the term bioactivity refers to a property of these new cements to form HA when immersed in vitro in a physiologic phosphate-buffered saline solution. Although significant in vitro data on the new bioactive cements exist, little-to-no clinical data are available. However, those clinical studies that have been done seem to be positive.15
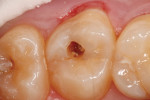
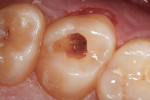
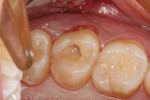
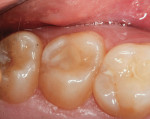
The clinical case depicted in Figure 3 through Figure 7 demonstrates the use of a bioactive liner and base in the treatment of a deep carious lesion. The patient presented with reversible pulpitis, which was characterized by sharp sensitivity to cold and biting and the brief painful response to stimuli. During excavation of caries, the clinician noted the cavity was close to the pulp with no pulpal exposure. The tooth has been asymptomatic for more than 3 years.
Potential Benefits to the Dentist
Bioactive materials offer noteworthy possibilities for dentistry, including the following:
• Bioactive restoratives have a remineralization and strengthening effect on human hard tissue, which is valuable for the treatment of acid-caused tooth-enamel erosion.
• The mineral enrichment efficacy leads to an immediate and long-lasting increase of the pH level. This will help protect tooth structure from the detrimental effects of all types of acids.
• These materials chemically bond to dentin. This property will also help decrease sensitivity typically caused by bonding technique errors.
• When activated with water, these materials release ions from their composition, forming a mineral matrix equivalent to that of natural HA.
• They are effective in reducing MMP formation, and thereby capable of decreasing, if not eliminating, the collagen breakdown commonly found in many resin–dentin bonding procedures.
• Bioactive glass is effective as an adjunct to conventional surgery in treatment of intrabony defects.
The benefits of these new products appear to be significant for both the patient and provider. The use of bioactive materials should result in a long-lasting restoration. Theoretically, it should also help to repair damaged dentin while decreasing the chance for recurrent caries.
Revisiting Bioactive Materials
Clinicians may need to reeducate themselves in the area of bioactive dental materials to fully comprehend their value and benefits for both providers and patients, especially for patients at high risk. Continuing education courses covering these materials would be beneficial, and educators should review materials options along with the evidence for or against their use. At a recent International Association for Dental Research meeting, a surprisingly substantial number of research papers were presented on this class of materials. The authors anticipate seeing many more companies introducing these types of products in the near future.
Return on investment with this “new” technology seems apparent. These materials should decrease the rate of recurrent caries, remineralize dentin, decrease sensitivity, help maintain better long-term bonded restorations, repair intrabony defects, create an apical plug during apexification, and help repair root perforations and improve the results for direct pulp caps. This new class of materials is potentially exciting for the future of restorative dentistry, as they appear to offer many benefits without high cost.
About the Authors
Gerard Kugel, DMD, MS, PhD
Professor and Associate Dean of Research
Tufts University School of Dental Medicine
Boston, Massachusetts
Steven Eisen, DMD
Associate Professor
Department of Comprehensive Care
Tufts University School of Dental Medicine
Boston, Massachusetts
References
1. Hench LL, Splinter RJ, Allen WC, Greenlee TK Jr. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5(6):117-141.
2. Hench LL. Bioactive ceramics: theory and clinical applications. In: Andersson OH, Happonen RP, Yli-Urpo A, eds. Bioceramics. Vol 7. Turku, Finland: Butterworth-Heinemann Ltd.; 1994:3-14.
3. Jeffries SR. Bioactive and biomimetic restorative materials: a comprehensive review. Part 1. Journal of Esthetic and Restorative Dentistry. 2014;26(1):14-26.
4. Randall RC, Wilson NH. Glass-ionomer restoratives: a systematic review of a secondary caries treatment effect. J Dent Res. 1999;78(2):628-637.
5. Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85(1):22-32.
6. Protroot® MTA Material Safety Data Sheet, Johnson City, TN: DENTSPLY Tulsa Dental Specialties; 2010. http://www.dentsplymea.com/sites/default/files/MSDS%20Proroot%20MTA%20Gray%201-28-10.pdf. Accessed April 4, 2016.
7. Sarkar NK, Caicedo R, Ritwik P, et al. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31(2):97-100.
8. Zhou HM, Shen Y, Wang ZJ, et al. In vitro cytotoxicity evaluation of a novel root repair material. J Endod. 2013;39(4):478-483.
9. Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29(5):580-593.
10. Chao W, Perry R, Kugel G. Surface deposition analysis of bioactive restorative material and cement [abstract 1313]. J Dent Res. 2016;95(spec iss A).
11. Doxa Dental AB. 510(k) Summary, XeraCem™, K081405, August 21, 2008. https://www.accessdata.fda.gov/cdrh_docs/pdf8/K081405.pdf. Accessed April 4, 2016.
12. Doxa Dental AB. 510(k) Summary, Ceramir® Crown & Bridge, K100510, March 25, 2010. https://www.accessdata.fda.gov/cdrh_docs/pdf10/K100510.pdf. Accessed April 4, 2016.
13. Lööf J, Svahn F, Jarmar T, et al. A comparative study of the bioactivity of three materials for dental applications. Dent Mater. 2008;24(5):653-659.
14. Murali S, Epstein N, Perry R, Kugel G. Fluoride release of bioactive restoratives with bonding agents [abstract 368]. J Dent Res. 2016;95(spec iss A).
15. Jefferies SR, Appleby D, Boston D, et al. Clinical performance of a bioactive dental luting cement—a prospective clinical pilot study. J Clin Dent. 2009;20(7):231-237.