Use of Titanium Mesh in Implant Site Development for Restorative-Driven Implant Placement: Case Report. Part 2—Surgical Protocol for Single-Tooth Esthetic Zone Sites
Abstract:
There are many techniques in the dental implant literature to augment bone for implant site development. The use of rigid titanium mesh (ti-mesh) was first described by Boyne in the mid-1980s to maintain regenerative space and to aid in unimpeded bone healing. Ti-mesh was used in this case report to demonstrate the predictability of this technique in creating bone augmentation in both a lateral and vertical direction prior to the placement of a single implant in site No. 5. The article describes the surgical steps for the use of ti-mesh in a single esthetic zone site with a 3-year follow-up.
An implant surgeon’s objective is to deliver a final result to his or her patients that restores them to a state of optimal health, function, and esthetics. Part of the surgeon’s task is to find the best and most predictable way to deliver this result. Unfortunately, not all cases are straightforward, and some may require additional procedures to achieve sufficient hard and soft tissue to place an implant in an ideal prosthetic position. The challenge is to not only employ techniques that will be predictable and successful, but also to minimize complications and morbidity for the patients.1-8
Various techniques are available to reconstruct deficient sites to permit implant placement; clinicians typically are most comfortable with certain techniques for treating different types of bony defects. This article will describe a step-by-step surgical approach of using a titanium mesh (ti-mesh) scaffold to predictably aid in reconstructing lost alveolar bone, and will discuss the preliminary results of a completed prospective case series (unpublished) on the efficacy of bone augmentation using ti-mesh for guided bone regeneration (GBR) in 62 consecutively treated patients with 77 individual ti-mesh scaffolds and 121 implants placed.
(Editor’s Note: Part 1 of this series, “Restorative Protocol for Single-Tooth Esthetic Zone Sites,” appeared in the April 2014 issue of Compendium. It can also be accessed online at dentalaegis.com/go/cced630.)
Hard tissue can be deficient as the result of several different phenomena, such as advanced periodontal disease, trauma, infection, traumatic extraction, or simply because of physiologic post-extraction resorption.1 Boyne introduced the concept of a titanium mesh scaffold as an alternative to traditional barrier membranes.9 The advantage was the ability to offer significantly increased space and continual space maintenance during the healing phase to allow bone regeneration to occur with fewer concerns about failure should the mesh barrier become exposed. Since that time, numerous studies have reported on the success of this technique to achieve significant osseous regeneration in implant site development procedures.10-19
Thus, the rationale for using a ti-mesh scaffold is that it allows for superior space maintenance while protecting the regenerative space and enables the regeneration of bone to occur with the help of osteogenic cells and growth factors from adjacent sites. The following case report demonstrates the surgical steps in the use of ti-mesh to achieve implant site development through ridge reconstruction in the esthetic zone replacing a hopeless maxillary right first premolar, tooth No. 5.
Case Report
Pre-Surgical Evaluation
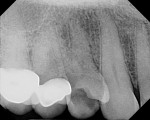
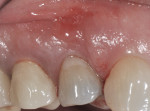
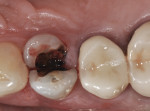
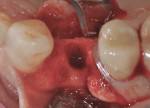
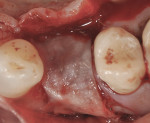
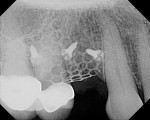
The patient presented as a 66-year-old white healthy, non-smoking (American Society of Anesthesiologists [ASA] 2) female with a clinical vertical root fracture and an acute buccal abscess associated with tooth No. 5 (Figure 1 and Figure 2). There were 5-mm interproximal and mid-buccal probing depths with a 2-degree mobility of the individual roots, which were separated from one another due to a clear vertical fracture to the osseous crest (Figure 3). The patient was uncomfortable and unable to chew on this tooth and had sharp pain when the “filling came out.” The tooth was deemed to have a hopeless prognosis, and a decision was made in coordination with the patient’s restorative dentist to replace the tooth with a dental implant. An esthetic risk assessment (ERA) was completed and a low-to-medium ERA profile was discussed with the patient.2-5,19
Based on the findings noted during the initial consultation it was explained to the patient that a staged approach would be the recommended course of therapy. Due to the loss of vertical height of bone on the facial aspect of the ridge, a GBR type of procedure was deemed necessary. It was decided to proceed with a bone grafting procedure using ti-mesh as the graft scaffold after allowing 8 weeks of soft-tissue healing post-extraction. This timeframe allows for soft-tissue closure over the extraction socket to increase the amount of keratinized gingiva for the bone-grafting procedure and aid in primary soft-tissue closure over the ti-mesh after its placement.19,20
Tooth Extraction
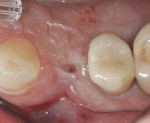
As the result of a vertical fracture, tooth No. 5 presented in two pieces, as each root was separated from the other. Each was removed with a simple forceps delivery without the need for any type of flap approach due to the hypermobility of each root segment. It was confirmed that the buccal plate of bone was totally missing at the apical third of the buccal root. The socket was thoroughly debrided to ensure removal of all granulation and infected tissue. This was accomplished using a 60-second rinse with povidone-iodine 10%, followed by 60-second flushes with sterile water and the use of a Piezosurgery® OT4 tip (Piezosurgery Incorporated, www.piezosurgery.us). A piece of collagen plug was placed into the socket to aid in clot stabilization, and the socket was closed overtop with two simple interrupted sutures of 4-0 chromic gut. The patient elected not to have any temporary tooth replacement during the time of healing due to her low lip-line esthetics, and the postoperative healing period was uneventful. At the 8-week post-extraction visit, the loss of significant buccal hard- and soft-tissue support for a future implant was documented (Figure 4).
GBR with Ti-Mesh
At 8 weeks post-extraction, a preoperative digital periapical radiograph and cone-beam computed tomography (CBCT) scan of site No. 5 showed that the buccal and palatal walls of bone were approximately 4 mm to 5 mm apical to the interproximal height of bone. Placement of an implant in this apico-coronal and buccal-lingual position would be too deep and in a poor palatal prosthetic position in comparison to the adjacent teeth and would lead to difficulties in the restorative phase. This would also compromise the long-term esthetics of the area due to the severe buccal concavity.
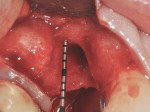
Sulcular incisions were made on the buccal and palatal aspects of tooth No. 6 with a crestal incision over the area of the previous No. 5. A distal vertical releasing incision was made to spare the papilla associated with tooth No. 4. A full-thickness flap was raised, and the area of the previous extraction socket was thoroughly curetted and debrided (Figure 5 and Figure 6) revealing the large residual bony ridge defect associated with the extraction of tooth No. 5. At the crestal aspect of the ridge, a 1 mm thickness of palatal bone remained, but all aspects of the facial plate were missing.
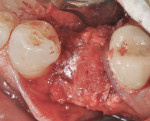
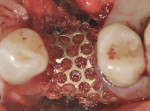
Intraosseous penetrations were made in the residual ridge with the use of Piezosurgery tip OP5 to induce a regional acceleratory phenomenon. A piece of room-temperature Regenaform® (Exactech, Inc., www.exac.com) was mixed with PRGF (platelet-rich growth factors) after taking the patient’s own drawn blood, which was spun-down to PRGF in an in-office centrifuge and mixed with calcium sulfate powder. The bone graft was then adapted into the residual defect to serve as an osteoconductive scaffold for bone regeneration (Figure 7). The bone graft material was then covered with the ti-mesh, which acts to secure the graft material in place (Figure 8 and Figure 9). Special attention must be paid to ensure that the ti-mesh is completely stabilized on all sides so that no micromovement of the mesh occurs during the healing phase. In the author’s experience, no less than three screws should be used to ensure adequate rigidity, and, preferably, additionally one in each corner. Once the ti-mesh was appropriately secured, a membrane was placed overtop for epithelial exclusion. Membrane exclusion is only used in cases where periosteal exclusion is not contraindicated, as with this case. A DynaMatrix® membrane (Keystone Dental, www.keystonedental.com) was soaked in the fraction-3 liquid from the patient’s PRGF and used to cover over the ti-mesh (Figure 10). Finally, a deep buccal periosteal releasing incision was performed to ensure tension-free primary closure of the surgical site. The area was subsequently sutured with a combination of horizontal mattress and simple interrupted sutures (Figure 11). A postoperative digital periapical radiograph (Figure 12) showed the three stabilizing screws and the ti-mesh graft in place.
Postoperative care was thoroughly reviewed with the patient, including medication dosages, which were to be continued until completion. One hour preoperatively, the patient had begun amoxicillin 500 mg, anaprox 550 mg, and a methylprednisolone dose pack.
Postoperative Healing and Implant Placement
The patient was followed-up at 2 weeks for suture removal and at 4, 8, and 12 weeks post-surgery to ensure appropriate healing and complete soft-tissue closure over the ti-mesh. An “early” exposure (< 3 months) may complicate the healing whereby a thicker pseudo-periosteum would be seen with less bone regeneration in the immediate site of exposure. It is unlikely that an exposure would occur if the tissues cover the mesh at 12 weeks. After that, individualized recall appointments should be scheduled to ensure continual monitoring of the site and to record whether any “late” exposure (> 3 months) of the ti-mesh is detected (Figure 13). While it is not uncommon for early or even late-stage exposures to occur, these exposures are rarely a cause for concern, as will be discussed later.
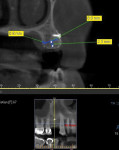
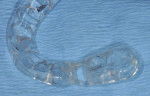
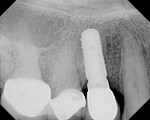
In this case, a CBCT scan was taken after 6 months of healing (Figure 14). The scan demonstrated significant regeneration of the facial plate, which would allow the implant No. 5 to be placed in an ideal prosthetic position. The scan showed a ridge width of 8 mm, with another 2.3 mm of vertical gain. The restorative dentist saw the patient soon after to fabricate a laboratory-made anatomically correct surgical guide template from newly taken impressions (Figure 15).
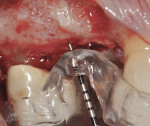
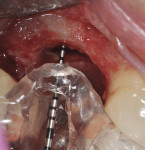
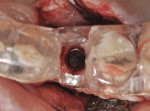
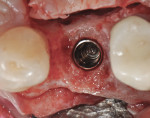
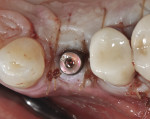
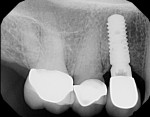
The patient was then scheduled for implant surgical placement at 7 months after the original GBR procedure. The re-entry confirmed that significant bone regeneration had occurred without incidence of ti-mesh exposure during the 7-month healing phase. The bone was noted to be Type III quality during osteotomy preparation. Bone scalloping of the ridge with an 8-round surgical length bur (ostectomy) was necessary due to the excellent vertical reconstruction, which was anticipated because the bone healed mesially-distally from the interproximal height of bone of the adjacent teeth (Figure 16). The implant osteotomy was positioned with the aid of the fabricated surgical guide template (Figure 17), and a Straumann 4.1-mm x 10-mm RC Bone Level implant (Straumann, www.straumann.us) was delivered (Figure 18) with excellent primary stability (> 35 Ncm insertion torque). A view of the final implant in-situ clearly demonstrates 3 mm of bone reconstruction buccal to the implant (Figure 19). An under-contoured 4-mm RC bottleneck healing abutment (Straumann) was placed to allow good primary interproximal soft-tissue closure and maximize tissue volume (Figure 20).
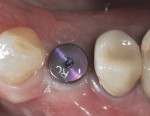
After 8 weeks of healing, a final postoperative visit confirmed bone healing with reverse torque testing at 35 Ncm using an implant carrier device and the manufacturer’s torque driver. The bottleneck healing abutment was now replaced with a conical healing abutment (Figure 21) to “stretch the tissues” for final impressions, which was coordinated with the restorative dentist. Typically, slight tissue blanching will be observed but will dissipate in a few minutes. The procedure was seamless for the patient, as the completion of the surgical phase was followed immediately by the commencement of the prosthetic phase with her restorative dentist. This team approach to dental implant care and coordinated visits between offices is very much appreciated by the authors’ patients. Closed-tray impressions were taken, a custom titanium abutment was fabricated by the dental laboratory to bring the cement margins to within 1.5 mm of the gingival margin, and the single crown was cemented with radiopaque zinc phosphate cement using the Teflon® tape, copy abutment technique.21,22
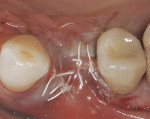
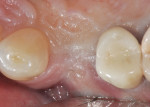
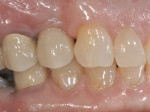
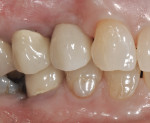
The patient will be followed yearly for 5 years as all periodontal maintenance visits are with her restorative dentist’s office. The 1-year clinical and digital periapical images are presented in Figure 22 and Figure 23, along with the 3-year documented results in Figure 24 and Figure 25. When comparing the 3-year and the 1-year results, it can be noted that the 3-year radiographic periapical crestal bone is more dense than at the 1-year time point, and also there is a noticeable filling in of the soft-tissue papillas interproximally at the later time point. (Restorative therapy was performed by Mark Krupnick, DDS, Philadelphia, Pennsylvania.)
Discussion
As demonstrated by this case report, the ti-mesh can be utilized to achieve significant amounts of bone regeneration in challenging bony defects. The predictability of this technique, however, cannot be demonstrated by a single case report. The authors of this study will be publishing their clinical and radiographic/CBCT findings of 62 patients treated by the primary author (RAL) in private practice with 77 ti-mesh, who have been followed to restorative completion. The data and statistics are expected to be completed by the end of 2014. Discussed here is an early compilation of the results thus far.
To date, 121 implants have been placed in reconstructed bone in 62 patients using the ti-mesh technique, with only one early failure (< 2 months), a 99.2% survival rate. Preliminary data shows the average gain in ridge width is 4.98 mm horizontally and 3.1 mm vertically. The one early failure was due to early mesh exposure and loss of the mesh and graft.
Several trends have been noted thus far during the course of this larger study of 77 ti-mesh cases. First, exposure of the mesh generally shows no signs of infection and does not appear to compromise the success rate of the procedure. A total of 19 exposures or a 24.7% rate (12 early [ie, < 3 months] and 7 late [ie, > 3 months]) were recorded, with the majority (11/19) observed in thin tissue biotypes. Implants were successfully placed in all cases of exposure except for one, and this patient was successfully rehabilitated with implants after a second ti-mesh procedure. What is observed upon ti-mesh removal in the exposed ti-mesh cases is that the thickness of the pseudo-periosteum layer9 under the mesh is increased and the bone quality appears to be lower in the area of exposure. Only one complete graft failure was recorded in an early exposure case, which was re-treated successfully. If exposure of the ti-mesh occurs during the healing period, it is important to closely follow-up and instruct the patient to apply chlorhexidine rinse locally to avoid infection in the area. Second, the use of biologic modifiers such as PRGF, Gem 21S® (Osteohealth Co., www.osteohealth.com), and Osteocel® (Ace Surgical Supply Co., Inc., www.acesurgical.com) appears to enhance the results of augmentation and lowers the rate of exposures. Third, of the 121 implants placed, 63 required additional bone grafting for “contour augmentation” (52%) with slowly resorbing bovine bone and a collagen membrane to provide additional thickness to the facial and to help ensure a long-term esthetic result with its corresponding soft- and hard-tissue maintenance.23-26
Conclusion
In conclusion, in this case report the ti-mesh GBR procedure proved to be highly predictable for augmenting bone in both a horizontal and vertical fashion. In the authors’ larger study of 62 patients with 77 ti-mesh and 121 implants placed, exposure of the ti-mesh showing no signs of infection did not compromise the success of the procedure and was much more common in patients with a thin biotype. Based on these findings of ti-mesh exposure, considerations should be given to augment the surgical site prior to the ti-mesh procedure with either autogenous bone graft, acellular dermal matrix, or Mucograft® (Geistlich, www.geistlich-mucograft.com). Not only does the long-term success of implants in the esthetic zone require a bone thickness of at least 2 mm to the facial of the placed implant, but a thickened soft-tissue base of keratinized gingiva should also be considered.25,26 All implants that were placed into exposed mesh sites (early or late) were successful. Furthermore, the use of certain biologic materials appears to enhance the soft- and hard-tissue healing results obtained with this technique.
Lastly, the need for “contour augmentation” in the larger study was noted to be 52% of the implant sites treated (63/121) to provide an implant of appropriate width in a good prosthetic position and with at least 2 mm of bone to the facial of the implant. Thus, in anticipation of this procedure the patient needs to be informed of the possibility of the need for further bone grafting at the time of implant placement to ensure the appropriate thickness of bone for long-term ethetic success.
References
1. Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants. 2009;24(suppl):237-259.
2. Martin W, Morton C, Buser D. Pre-operative analysis and prosthetic treatment planning in esthetic implant dentistry. In: Buser D, Belser U, Wismeijer D, eds. ITI Treatment Guide, Vol 1: Implant Therapy in the Esthetic Zone for Single-Tooth Replacements. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2007:9-24.
3. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants. 2004;19(suppl):43-61.
4. Levine RA, Nack G. Team treatment planning for the replacement of esthetic zone teeth with dental implants. Compend Cont Educ Dent. 2011;32(4):44-50.
5. Levine R, Martin W. Esthetic risk assessment in implant dentistry. Inside Dentistry. 2012;8(8):66-71.
6. Belser UC, Schmid B, Higginbottom F, Buser D. Outcome analysis of implant restorations located in the anterior maxilla: a review of the recent literature. Int J Oral Maxillofac Implants. 2004;19(suppl):30-42.
7. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25(2):113-119.
8. Buser D, Belser U, Wismeijer D, eds. ITI Treatment Guide, Vol 1: Implant Therapy in the Esthetic Zone for Single-Tooth Replacements. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2007:1-24.
9. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87-91.
10. von Arx T, Kurt B. Implant placement and simultaneous peri-implant bone grafting using a micro titanium mesh for graft stabilization. Int J Periodontics Restorative Dent. 1998;18(2):117-127.
11. von Arx T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10(1):24-33.
12. Artzi Z, Dayan D, Alpern Y, Nemcovsky CE. Vertical ridge augmentation using xenogenic material supported by a configured titatnium mesh: clinicohistopathologic and histochemical study. Int J Oral Maxillofac Implants. 2003;18(3):400-406.
13. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237-247.
14. Longoni S, Sartori M, Apruzzese D, Baldoni M. Preliminary clinical and histologic evaluation of a bilateral 3-dimensional reconstruction in an atrophic mandible: a case report. Int J Oral Maxillofac Implants. 2007;22(3):478-483.
15. Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18(3):286-294.
16. Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. Reconstruction of the maxilla and mandible with particulate bone graft and titianium mesh for implant placement. J Oral Maxillfac Surg. 2008;66(2):235-245.
17. Misch CM. Bone augmentation of the atrophic posterior mandible for dental implants using rhBMP-2 and titanium mesh: clinical technique and early results. Int J Periodontics Restorative Dent. 2011;31(6):581-589.
18. Her S, Kang T, Fien MJ. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012;70(4):803-810.
19. Levine RA, Manji A, Faucher J, Present S. Use of titanium mesh in implant site development for restorative-driven implant placement: case report. Part 1—restorative protocol for single-tooth esthetic zone sites. Compend Contin Educ Dent. 2014;35(4):264-273.
20. Chen ST, Buser D. Clinical and esthetic outcomes of implants placed in postextraction sites. Int J Oral Maxillofac Implants. 2009;24(suppl):186-217.
21. Linkevicius T, Vindasiute E, Puisys A, Peciuliene V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin Oral Implants Res. 2011;22(12):1379-1384.
22. Present S, Levine RA. Techniques to control or avoid cement around implant retained restorations. Compend Contin Educ Dent. 2013;34(6):432-437.
23. Levine R. Implant site preparation: horizontal ridge augmentation using particulate allograft and the principles of guided bone regeneration. In: Sonick M, Hwang D, eds. Implant Site Development. 1st ed. Hoboken, NJ: Wiley-Blackwell; 2011:179-201.
24. Buser D, Wittneben J, Bornstein MM, et al. Stability of contour augmentation and esthetic outcomes of implant-supported single crowns in the esthetic zone: 3-year results of a prospective study with early implant placement postextraction. J Periodontol. 2011;82(3):342-349.
25. Levine RA, Huynh-Ba, Cochran DL. Soft tissue augmentation procedures for mucogingival defects in esthetic sites. Int J Oral Maxillofac Implants. 2014;29(suppl):155-185.
26. Morton D, Chen ST, Martin WC, et al. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. 2014;29(suppl):216-220.
About the Authors
Robert A. Levine, DDS, FCPP
Clinical Professor in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania;
Private Practice, Pennsylvania Center for Dental Implants and Periodontics, Philadelphia, Pennsylvania;
Diplomate, American Board of Periodontology; Fellow, International Team for Implantology, Basel, Switzerland
Aleem Manji, DDS, MS
Former Postgraduate Resident in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania;
Private Practice, Toronto, Canada;
Diplomate, American Board of Periodontology; Fellow, Royal College of Dentists of Canada
Joanie Faucher, DMD, MS
Professor, Department of Periodontology, Laval University, Quebec City, Canada;
Former Postgraduate Resident in Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania;
Private Practice, Quebec, Canada;
Diplomate, American Board of Periodontology; Fellow, Royal College of Dentists of Canada
Philip Fava, DMD, MDSc
Former Postgraduate Resident in Periodontology and Implantology, University of Connecticut School of Dental Medicine, Hartford, Connecticut;
Private Practice, Pennsylvania Center for Dental Implants and Periodontics, Philadelphia, Pennsylvania;
Member, International Team for Implantology, Basel, Switzerland