Mineral Trioxide Aggregate: Part 2 – A Review of the Material Aspects
Neeraj Malhotra, MDS, PGDHHM; Antara Agarwal, MDS; and Kundabala Mala, MDS
Abstract
The purpose of this two-part series is to review the composition, properties, and products of mineral trioxide aggregate (MTA) materials. PubMed and MedLine electronic databases were used to identify scientific papers from January 1991 to May 2010. Based on the selected inclusion criteria, citations were referenced from the scientific peer-reviewed dental literature. Mineral trioxide aggregate is a refined form of the parent compound, Portland cement (PC), and demonstrates a strong biocompatibility due to the high pH level and the material’s ability to form hydroxyapatite. Mineral trioxide aggregate materials provide better microleakage protection than traditional endodontic materials as observed in findings from dye-leakage, fluid-filtration, protein-leakage, and bacterial penetration-leakage studies and has been recognized as a bioactive material. Various MTA commercial products are available, including gray mineral trioxide aggregate (GMTA), white mineral trioxide aggregate (WMTA), and mineral trioxide aggregate-Angelus (AMTA). Although these materials are indicated for various dental uses and applications, long-term in-vivo clinical studies are needed. Part 1 of this article highlighted and discussed the composition and characteristics of the material. Part 2 provides an overview of commercially available MTA materials.
Mineral trioxide aggregate (MTA) was developed at Loma Linda University (Loma Linda, California, USA) and was first described in the dental scientific literature in 1993.1-3 The chemical composition of MTA was determined by Torabinejad et al.4 It received US Food and Drug Administration approval in 1998.1,2 As discussed in part 1 of this article, MTA possesses most of the required characteristics of an ideal endodontic material, which are:
• a pH similar to calcium hydroxide (12.5)3
• a compressive strength equivalent to intermediate restorative material (IRM) and Super EBA4
• less microleakage and better sealing ability than amalgam, zinc oxide-eugenol (ZOE), IRM, and conventional glass ionomer4,5
• biocompatibility5
• antibacterial and antimicrobial actions6
• regenerative potential with biologic activity3,5
• minimal solubility7
• radiopacity3,4
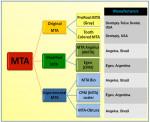
A variety of MTA products are on the market. The first commercially available product was gray mineral trioxide aggregate (GMTA), marketed as ProRoot® MTA (DENTSPLY Tulsa Dental, www.tulsadentalspecialties.com). To decrease the potential for tooth discoloration observed when GMTA is used in anterior teeth, an alternative formulation, known as tooth-colored MTA or white mineral trioxide aggregate (WMTA) (DENTSPLY, www.denstply.com), was developed. Both products have demonstrated similar physicochemical and biologic properties, differing mainly in chemical composition.6 In 2001, MTA-Angelus (Angelus Indústria de Produtos Odontológicos S/A, www.angelus.ind.br) was introduced. The color of this product was then changed to white and marketed as MTA Branco (MTAB) (Angelus Indústria de Produtos Odontológicos S/A). Part 2 of the review discusses MTA materials and briefly compares MTA with Portland cement (PC).
Methodology
An electronic search was conducted in PubMed and MedLine with appropriate medical subject headings (MeSH). The search terms (keywords/headings) used were: mineral trioxide aggregate (MTA); White MTA (WMTA); Gray MTA (GMTA); MTA Angelus (AMTA); and Portland cement (PC). An additional search was also done using the terms pulp capping agents; retro-filling materials; perforation repairs (lateral and furcation); root-end filling materials; newer obturation materials; apexogenesis; and apexification. Only articles relevant to the topic (MTA) and published in English in peer-reviewed journals from January 1991 to May 2010 were included. Following this, a hand-search was conducted for the available issues of all the major journals pertaining to the topic.
Types of Products
The following is a discussion of MTA products on the market (Figure 1).
Gray Mineral Trioxide Aggregate
Gray mineral trioxide aggregate (GMTA), the traditional variety, was first on the market (ProRoot MTA, DENTSPLY-Tulsa Dental) following the introduction of MTA. The product has particle sizes of 1 µm to 10 µm and exhibits significantly higher initial and final setting times, greater compressive strength, and less microleakage (as an apical barrier) as compared with white mineral trioxide aggregate (WMTA).8,9 When used to repair furcation perforations, GMTA provides an equivalent protection as a ZOE preparation.3 GMTA shows more microleakage than laterally condensed and thermoplasticized gutta percha but less apical microleakage than warm, vertically condensed gutta percha.3 Immediately after mixing, GMTA exhibits a higher pH level than those of WMTA and PC. However, after 60 minutes, pH levels decrease significantly and are lower than those of WMTA and PC.6 Gray mineral trioxide aggregate is equally effective at lower concentrations as WMTA against Enterococcus faecalis and Streptococcus sanguinis.10 Similarly, at lower concentrations, GMTA is observed to be more effective against Candida albicans as compared with WMTA; however, at concentrations of 50 mg/mL and 25 mg/mL, both are equally inhibitive for 7 days.11,12 Periodontal ligament fibroblasts, gingival fibroblasts, and osteoblasts have been shown to exhibit good adhesion/attachment, spreading, and growth on GMTA surfaces.4,5 Also, in vivo studies have shown GMTA to have minimal inflammation and tissue regeneration.
White Mineral Trioxide Aggregate
White mineral trioxide aggregate was introduced as white ProRoot MTA/Tooth-Colored MTA (DENTSPLY, www.dentsply.com) to address esthetic concerns associated with the use of traditional GMTA.4 The composition of WMTA is almost identical to that of GMTA, except for the absence of iron compound (tetracalcium aluminoferrite) in WMTA.6,13-15 It contains 54.9% less aluminium oxide, 56.5% less magnesium oxide, and 90.8% less ferrous oxide as compared with GMTA. Thus, reduction in the iron compound (ferrous oxide) is most likely the main reason for the lighter color change, with reduction in magnesium acting as a contributing factor. White mineral trioxide aggregate has an overall smaller particle size (8 times smaller) and narrower range of size distribution than GMTA,16 which attributes to its better handling characteristics and compatibility than GMTA. It demonstrates significantly more solubility than GMTA.9 Presence of other substances apart from bismuth oxide may be responsible for its better radiopacity than GMTA.4,9 White mineral trioxide aggregate exhibits greater hardness, less solubility, and more radiopacity than PC.4,6
Results from cell culture and cell attachment methods have shown good biocompatibility of WMTA, with biocompatibility comparable with that of GMTA. In extended culture, the cells form an extensive matrix-like layer on the WMTA surface similar to that observed on the surface of GMTA.17,18 White mineral trioxide aggregate has no genotoxic effects and has the potential to induce osteogenesis. Examinations have demonstrated subcutaneous implantation of dentin tubes filled with WMTA have similar tissue changes and bridge formation as observed with GMTA, indicating similar mechanisms of action.5 White mineral trioxide aggregate has shown to have a stimulating effect on human dental pulp cells. Results from pulp-capping tests have also shown no difference between GMTA and WMTA. Both agents tend to form complete dentin bridges and hard tissue barriers with minimal tissue inflammation.5,19 White mineral trioxide aggregate can induce a general osteogenic phenotype in periodontal ligament fibroblasts, with induction of alkaline phosphatase activity, as well as production of osteonidogen, osteonectin, and osteopontin.20
Contrasting results are available regarding the comparative sealing abilities of GMTA and WMTA.21,22 Some recent investigations have shown that 24-hour set GMTA demonstrates significantly less leakage than white MTA, suggesting GMTA is a better sealing agent than WMTA. Other studies have shown no significant differences between WMTA and GMTA regarding sealing ability and microleakage (dye-leakage and bacterial-leakage tests) in cases of furcation perforation repair (orthograde and retrograde directions) and root canal obturation. The antibacterial activity of WMTA is similar to ZOE preparations against Staphylococcus aureus, E. faecalis, and Pseudomonas aeruginosa.4 Also, use of 0.12% chlorhexidine gluconate preparations provide more antibacterial activity against Actinomyces odontolyticus, Fusobacterium nucleatum, S. sanguis, E. faecalis, Escherichia coli, S. aureus, P. aeruginosa, and C. albicans as compared to the use of sterile water alone.4,23
MTA-Angelus
Available in grey (AGMTA) and white (AWMTA), MTA-Angelus (Angelus Solucoes Odontologicas) is silicate cement mainly consisting of 80% PC and 20% bismuth oxide.13,24 Gray mineral trioxide aggregate has more homogenous chemical composition and particle sizes compared with AGMTA. It contains a lower content of bismuth oxide and magnesium phosphate and a greater amount of calcium carbonate, calcium silicate, and barium zinc phosphate than GMTA.13 As opposed to GMTA, it shows presence of aluminum and has no iron.24 Although the amount of calcium is higher in AMTA, it exhibits only slightly higher pH levels and calcium release than GMTA.6 Like GMTA, AMTA has a stimulant effect on the cell growth of human gingival cells. Both MTA-Angelus products exhibit no cytotoxicity and genotoxicity on various cell lines, with effects similar to MTA on cell cultures.5 A dye-leakage study showed that MTA Angelus has less microleakage than amalgam, Vitremer™ (3M ESPE, www.3MESPE.com), and Super EBA25 with no difference in leakage between AWMTA and WMTA when used as apical barrier for open-apex teeth.26 However, GMTA has performed better than AMTA as a perforation repair material.5 MTA-Angelus has a lower radiopacity than WMTA and GMTA.9 Absence of dehydrated calcium sulfate makes the material setting time 10 to 15 minutes (14.28 + 0.49 min), which is lower than setting times of WMTA and GMTA.27 Both AGMTA and AWMTA have shown antimicrobial activity against Micrococcus luteus, S. aureus, E. coli, P. aeruginosa, C. albicans, and E. faecalis.28 MTA-Angelus has shown successful healing in cases of pulpotomy, pulp capping, internal resorptive defects, and root perforation, with properties making this product suitable for nonsurgical root canal obturation.29,30
Portland Cement
Portland cement (PC) forms the material basis for MTA. A mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, and tetracalcium aluminoferrite,15,31 PC is available in gray and white.6 In the case of white cement, a fluxing agent is used to remove the ferrite phase and produce the white version. Findings from a number of studies have unequivocally shown that the chemical composition of PC is similar to that of MTA, if not identical.6,13 Portland cement differs from MTA by the absence of bismuth ions and presence of potassium ions. Like MTA, PC produces calcium silicate hydrate gel and calcium hydroxide on hydration. Cell culture studies of endothelial, L929 fibroblast, and human osteosarcoma cells reveal no significant differences between PC and MTA.6 Viability of human periodontal ligament cells with PC is similar to MTA at 12 and 24 hours.32 No observable genotoxic effects of PC have been reported.33 Camilleri et al34 assessed the biocompatibility of grey and white PCs, grey and white mineral trioxide aggregate, and accelerated PC, produced by excluding gypsum from the manufacturing process and their eluants. The cement eluants (consisting of calcium hydroxide) of grey or white MTA and accelerated PC do not show the presence of any toxic leachables along with induction of cell proliferation.34 However, poor cellular growth was observed when the material was seeded in direct contact with the test cements. The efficacy of PC for pulp capping is also comparable with that of MTA.35
Although PC and MTA have similar compositional characteristics, they do not have similar structural features and properties (ie, setting expansion, compressive strength, and radiopacity).6 Mineral trioxide aggregate commercial products have smaller mean particle sizes, contain fewer toxic heavy metals, have longer working times, and undergo additional processing/purification than regularly available PCs.4,9
Chemical constitution, radiopacity, and biocompatibility of an experimental PC-containing bismuth oxide have also been tested and have shown contrasting results. The constitution of the material is similar to MTA with more irregular and larger particles than MTA.36 The radiopacity and biocompatibility (MTT assay and tissue reactions) of the experimental cement is observed to be similar to MTA.36,37 However, other studies observed that the addition of bismuth oxide to PC powder at all ratios significantly lowers the cell viability of human periodontal ligament cells.32 Moreover, increasing amounts of bismuth oxide has been shown to increase the porosity, solubility, flaws, cracks, and degradation of the material.
Results from characterization studies have indicated PC cannot be recommended as a suitable substitute of MTA for the following reasons6:
• The quality and composition is difficult to predict. Studies have shown that the arsenic content of PC is 6 times the amount of arsenic in GMTA.
• The high solubility can lead to release of a toxic element and early degradation of the material, jeopardizing the long-term safety of PC.
• Carbon dioxide in inflamed tissues readily reacts with available water to form carbonic acid, which reacts with the cement to form calcium hydrogen carbonate. This carbonation decreases the tensile strength and resiliency of the material, leading to crack formation and buckling under high stress. The compressive strength of some types of PC is also observed to be lower than MTA.
• As opposed to PC, MTA is manufactured in laboratories as a medical material and is approved by the U.S. Food and Drug Administration for use in humans.
Although MTA is considered a refined form of PC material (parent compound), substitution with PC for MTA is discouraged for clinical purposes. Generally, researchers do not suggest that MTA and PC have the same clinical, biologic, and mechanical properties and do not consider PC to be a suitable replacement for MTA as an endodontic material.38 More clinical studies, especially long-term, are necessary before PC can be recommended as a substitute for MTA as an endodontic material.
Others
The products CPM (in white) and Endo-CPM sealer (Egeo S.R.L., www.egeodental.com.ar) were introduced in Argentina as MTA and MTA root canal sealer, respectively.6 The composition CPM sealer is similar to MTA, except for the presence of calcium carbonate for reducing the pH level of the material. ProRoot Endo Sealer (DENTSPLY Tulsa Dental Specialties) has a higher push-out strength as compared with AH Plus® (DENTSPLY Middle East and Africa, www.dentsplymea.com) and Pulp Canal SealerTM (Sybron Dental Specialties Inc., www.sybronendo.com) and exhibits significantly less leakage in comparison with Pulp Canal Sealer.39
Clinical Applications
Mineral trioxide aggregate materials are indicated for various restorative, endodontic, and regenerative dental procedures, such as vital pulp therapy, apexification, perforation repair (lateral and furcation), root-end filling, internal bleaching, and resorption repair, and as an obturating material (partial or complete) (Figure 2).1,2,4,30,40-43 Most research has focused on GMTA and WMTA. Findings from the available literature show MTA materials have favorable results and are materials of choice for the indicated clinical applications. However, data from well-designed human trials are still lacking for these applications and further clinical studies are needed to confirm efficacy as compared with other endodontic materials. Similarly, the available data for various clinical applications of AMTA are limited, and further clinical trials and studies are required.
Conclusion
The ideal study to examine the effects of any material or product is to conduct human clinical trials. Due to the obvious ethical constraints and issues involved with conducting such trials, animal studies are being performed to provide the required valuable information about commercial products. However, extrapolation of animal study results cannot necessarily be applied to humans and should be undertaken with caution. Overall, results in human studies conducted to date have shown positive results with MTA. However, further longitudinal studies are still required. Thus, well-designed randomized clinical trials and controlled clinical studies are needed to conduct a systematic and meta-analysis review of MTA materials, for all indicated uses for products on the market.
Editor's Note: To read Part 1 of this series, click here.
References
1. Rao A, Rao A, Shenoy R. Mineral trioxide aggregate—a review. J Clin Pediatr Dent. 2009;34(1):1-7.
2. Schwartz RS, Mauger M, Clement DJ, Walker WA III. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999;130(7): 967-975.
3. Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008; 24(2):149-164
4. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995(7);21:349-353.
5. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190-202.
6. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
7. Herzog-Flores DS, Andrade VLM, Méndez GV, et al. Physical chemical analysis of mineral trioxide aggregate (MTA) by X-rays diffraction, colorimetry and electronic microscopy. Rev ADM. 2000;17:125-131.
8. Matt GD, Thorpe JR, Strother JM, McClanahan SB. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one- or two-step apical barrier technique. J Endod. 2004;30(12):876-879.
9. Islam I, Chng HK, Yap HU. Comparison of physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32(3);193-197.
10. Al-Hezaimi K, Al-Shalan TA, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Anti-bacterial effect of two mineral trioxide aggregate preparations against Enterococcus faecalis and Streptococcus sanguis in vitro. J Endod. 2006;32(11):1053-1056.
11. Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J Endod. 2005;31(9):684-668.
12. Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Comparison of anti-fungal activity of white-colored and gray-colored mineral trioxide aggregate at similar concentrations against Candida albicans. J Endod. 2006;32(4):365-367.
13. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white Pro Root MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):809-815.
14. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31(2):101-103.
15. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21(8):731-738.
16. Asgary S, Parirokh M, Eghbal MJ, Stowe S, Brink F. A qualitative X-ray analysis of white and grey mineral trioxide aggregate using compositional imaging. J Mater Sci Mater Med. 2006;17(2):187-191.
17. Perinpanayagam H. Cellular response to mineral trioxide aggregate root-end filling materials. J Calif Dent Assoc. 2009;75(5):369-372.
18. Al-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006;32(9):872-875.
19. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32(7):601-623.
20. Bonson S, Jeansonne BG, Lallier TE. Root-end filling materials alter fibroblast differentiation. J Dent Res. 2004;83(5):408-413.
21. Hamad HA, Tordik PA, McClanahan SB. Furcation perforation repair comparing gray and white MTA: a dye extraction study. J Endod. 2006;32(4):337-340.
22. Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Human saliva penetration of root canals obturated with two types of mineral trioxide aggregate cements. J Endod. 2005;31(6):453-456.
23. Stowe TJ, Sedgley CM, Stowe B, Fenno JC. The effects of chlorhexidine gluconate (0.12%) on the antimicrobial properties of tooth-colored ProRoot mineral trioxide aggregate. J Endod. 2004;30(6):429-431.
24. Oliveira MG, Xavier CB, Demarco FF, Pinheiro AL, Costa AT, Pozza DH. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007;18(1):3-7.
25. Pereira CL, Cenci MS, Demarco FF. Sealing ability of MTA, Super EBA, Vitremer and amalgam as root-end filling materials. Braz Oral Res. 2004;18(4):317-321.
26. Lolayekar N, Bhat SS, Hegde S. Sealing ability of ProRoot MTA and MTA-Angelus simulating a one-step apical barrier technique—an in vitro study. J Clin Pediatr Dent. 2009;33(4):305-310.
27. Santos AD, Araújo EB, Yukimitu K, Barbosa JC, Moraes JC. Setting time and thermal expansion of two endodontic cements. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(3):e77-e79.
28. Tanomaru-Filho M, Tanomaru JM, Barros DB, Watanabe E, Ito IY. In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J Oral Sci. 2007;49(1):41-45.
29. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
30. Hashem AA, Hassanien EE. ProRoot MTA, MTA-Angelus and IRM used to repair large furcation perforations: sealability study. J Endod. 2008;34(1):59-61.
31. Sarkar NK, Caidedo R, Tirwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31(2):97-100.
32. Kim EC, Lee BC, Chang HS, Lee W, Hong CU, Min KS. Evaluation of the radiopacity and cytotoxicity of Portland cements containing bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(1):e54-e57.
33. Braz MG, Camargo EA, Salvadori DM, Marques ME, Ribeiro DA. Evaluation of genetic damage in human peripheral lymphocytes exposed to mineral trioxide aggregate and Portland cements. J Oral Rehabil. 2006;33(3):234-239.
34. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005;38(11):834-842.
35. Holland R, de Souza V, Murata SS, et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J. 2001;12(2):109-113.
36. Hwang YC, Lee SH, Hwang IN, et al. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):e96-e102.
37. Coutinho-Filho T, De-Deus G, Klein L, Manera G, Peixoto C, Gurgel-Filho ED. Radiopacity and histological assessment of Portland cement plus bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):e69-e77.
38. Steffen R, van Waes H. Understanding mineral trioxide aggregate/Portland-cement: a review of literature and background factors. Eur Arch Paediatr Dent. 2009;10(2):93-97.
39. Huffman BP, Mai S, Pinna L, et al. Dislocation resistance of ProRoot Endo Sealer, a calcium silicate-based root canal sealer, from radicular dentine. Int Endod J. 2009;42(1):34-46.
40. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197-206.
41. Schmitt D, Lee J, Bogen G. Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent. 2001;23(4):326-330.
42. Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35(6):777-790.
43. Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives—permanent teeth. J Endod. 2008;34(7 Suppl):S25-S28.
About the Authors
Neeraj Malhotra, MDS, PGDHHM
Reader
Department of Conservative Dentistry & Endodontics
KDDC, Mathura, U.P., India
Antara Agarwal, MDS
Senior Dental Consultant, Cosmetic Dentistry & Endodontics
Indus Hygiea
Unit of Indus Speciality Health
Mohali, Punjab, India
Kundabala Mala, MDS
Professor
Department of Conservative Dentistry and Endodontics
Manipal College of Dental Sciences
Mangalore, Karnataka, India