Mineral Trioxide Aggregate: A Review of Physical Properties
Neeraj Malhotra, MDS, PGDHHM; Antara Agarwal, MDS; and Kundabala Mala, MDS
ABSTRACT
The purpose of this two-part series is to review the composition, properties, products, and clinical aspects of mineral trioxide aggregate (MTA) materials. Electronic search of scientific papers from January 1991 to May 2010 was accomplished using PubMed and MedLine search engines to include relevant scientific citations from the peer-reviewed journals published in English. MTA is a refined form of the parent compound, Portland cement (PC). It demonstrates a strong biocompatible nature owing to the high pH and its ability to form hydroxyapatite. MTA materials provide a better seal than traditional endodontic materials as observed in dye leakage, fluid filtration, protein leakage, and bacterial penetration leakage studies, and it has been recognized as a bioactive material. Currently a variety of MTA commercial products are available, including Proroot® Gray MTA and White MTA both from DENTSPLY Tulsa Dental Specialties (www.DENTSPLY.com), and MTA Angelus (Angelus, www.angelus.ind.br). Although these materials are indicated for various dental uses/applications, long-term in-vivo clinical studies are still needed to claim the same. This first of this series highlights and discusses the composition, physical, and/or chemical properties of MTA. A subsequent article will offer an overview of the material aspect (commercial products) and clinical considerations for MTA materials.
Endodontic failures may occur as a result of leakage of irritants into the periapical tissues.1 Therefore, an ideal orthograde and/or retrograde filling material should seal the pathways of communication between the root canal system and its surrounding tissues; thus, this material should be biocompatible and dimensionally stable.2,3 This led to the development of mineral trioxide aggregate (MTA) materials possessing these ideal characteristics. The initial literature regarding the material was published in 1993 by Lee et al.4 Following this, the material received Food and Drug Administration (FDA) approval in 1998.5,6 Initially recommended as a root-end filling material, it is currently being used for pulp capping, pulpotomy, apexogenesis and apexification, apical barrier formation, repair of root perforations and resorptive defects, and as a root canal and root-end filling material.6,7 It is mainly composed of tricalcic silicate, tricalcic aluminate, and bismuth oxide, and consists of fine hydrophilic particles that harden in the presence of dampness or blood.5,6,8 It has a better sealing capacity and biocompatibility compared to other classic materials such as amalgam, cements, super ethoxy benzoic acid (EBA), and interim restorative material (IRM). This review highlights the compositional characteristics and featured properties of MTA materials.
Methodology
An electronic search of scientific papers was accomplished using PubMed and MedLine search engines and Cochrane databases using selected keywords and with appropriate medical subject headings (MeSH). The search terms (keywords/headings) used were: mineral trioxide aggregate (MTA); White MTA (WMTA); Gray MTA (GMTA); MTA Angelus (AMTA); Portland cement (PC); properties of MTA (physical, chemical, bacterial, biological, biocompatibility); pulp capping agents; retro-filling materials; perforation repairs (lateral and furcation); root-end filling materials; recent advances in endodontic materials; newer obturation materials; apexogenesis; and apexification. Only articles relevant to the topic (MTA) and published in English in peer-reviewed journals from January 1991 to May 2010 were included. Following this, a hand-search was conducted for the available issues of all the major journals pertaining to the topic.
Composition
MTA is an ash-colored powder made up of fine hydrophilic particles.6,10 Available as Gray MTA (GMTA) and White MTA (WMTA), both formulae basically are 75% Portland cement, 20% bismuth oxide, and 5% gypsum (Ca) by weight.11,12 Thus, MTA is a mixture of a refined Portland cement and bismuth oxide (17% to 18%) with trace amounts of SiO2, CaO MgO, K2SO4, and Na2SO4.5,13 Bismuth oxide is added to make the material radiopaque. Bismuth affects calcium hydroxide precipitation after MTA hydration; and under acidic conditions (inflammation), bismuth oxide can be released in the environment decreasing MTA’s biocompatibility as it inhibits cell proliferation.14,15 Gray MTA (GMTA) principally consists of tricalcium silicate, dicalcium silicate, tricalcium oxide, tricalcium aluminate, tetracalcium aluminoferrite, calcium sulphate, silicate oxide, and bismuth oxide,12,13 with a predominance of calcium and phosphorus ions (as per earlier reports).16 However, recent investigations using electron probe microanalysis suggested that phosphorus levels in MTA products are very low.17,18 White MTA (WMTA) basically lacks the tetracalcium aluminoferrite component with a lesser quantity/content of iron, aluminium, and magnesium oxides.12,17 Another commercially available MTA material is MTA-Angelus, which is 80% Portland cement and 20% bismuth oxide, and is more radiopaque than GMTA.12
Manipulation and Setting
MTA is available either as a box of five 1-gram single-use packets or as premeasured water packs for easy manipulation and application. ProRoot liquid microampules (sterile water) and a carrier are also provided with the packet. It should be stored in closed sealed containers away from moisture.5,10
The powder is mixed with supplied sterile water in a 3:1 powder/liquid ratio. A paper pad or a glass slab and a plastic or a metal spatula is used to mix the material to obtain a putty-like consistency. The mixing time should be less than 4 minutes, as prolonged mixing can cause dehydration of the mixture.10,19 The mixture can be carried with a plastic or metal carrier.20 The unused portion of MTA powder can be stored in sterilized empty film canisters.
MTA is uninhibited by blood or water, as moisture is required for a better setting of the material.21 The required hydration for setting is provided by a moist cotton pellet placed temporarily (until the next appointment) in direct contact and/or on the surrounding tissues.22 The hydration reaction during setting occurs between tricalcium silicate (3CaO·SiO2) and dicalcium silicate (2CaO·SiO2) to form a calcium hydroxide and calcium silicate hydrate gel, producing an alkaline pH.12,20,23 However, Dammaschke et al reported that calcium hydroxide is a product of tricalcium aluminate hydrogenation.12,24 A further reaction forms a high-sulphate calcium sulphoaluminate during the reaction with tricalcium aluminate and calcium phosphate.25 The released calcium ions diffuse through dentinal tubules, and increase their concentration over time as the material cures.26 Upon hydration, the poorly crystallized and porous solid gel (hydrated forms of components)14 that is formed solidifies to a hard structure in approximately 3 to 4 hours (initial set), with mean setting time of 165 ± 5 minutes.8,27,28 Although moisture is needed for setting of the material, excess moisture can result in a soupy mix that is difficult to use.5
The hydrated set material consists of interlocked cubic and needle-like crystals. The needle-like crystals exist as sharply delineated thick bundles filling the inter-grain space between the cubic crystals.13,29 MTA retention and push-out strength increase with time, extending from 72 hours to 21 days, indicating a prolonged maturation process of the material.10,13,19 This slower setting time may reduce the setting shrinkage, contributing to the low microleakage shown by the material. X-ray photoelectron spectroscopy (XPS) examination of WMTA has reported a threefold increase in surface sulphur and potassium species during the setting reaction.24 Thus, a passivating trisulphate species layer may aid in prolonging the setting time of the material (WMTA) and also serve a protective function.8,24
Thus, one of the main drawbacks of MTA is the extended setting period and the prolonged maturation phase. Use of different liquids and additives for the manipulation of MTA powder can influence the setting time and compressive strength of the material.13,30 However, it is imperative that the manipulation liquid have adequate water content with necessary diffusion ability to allow the hydration reaction to occur.13 Calcium chloride solutions (3% to 5%) and sodium hypochlorite gels decrease the setting time, whereas saline and 2% lidocaine increase the setting time. Chlorhexidine gluconate affects the surface hardness of MTA (WMTA) during the initial 24 hours.31 However, use of calcium chloride and sodium hypochlorite reduce the final compressive strength (as compared to sterile water) with saline and 2% lidocaine, having no significant affect on it.30 Accelerators such as sodium phosphate dibasic (Na2HPO4) also reduce the setting time.5,30
Usually, MTA is pressed into the desired location and not really condensed. The mixture is usually condensed with a moistened cotton pellet using light gentle strokes.5 Either hand or ultrasonic instruments can be used for placement and/or condensation of MTA. Hand instruments like Tulsa Carrier (DENTSPLY Tulsa Dental Specialties, www.dentsply.com), amalgam carrier, pluggers, paper points, messing gun MTA carriers, large bore needles, or Lentulo spirals can be used.5,8,10 In ultrasonic condensation, a hand instrument (condenser) placed in direct contact with MTA is activated by ultrasonics placed in contact with the shaft of the hand instrument. Different results are obtained from different studies regarding the best condensation technique for placement of MTA. Some authors claimed that hand condensation techniques offer less porosity and better adaptation than ultrasonic-assisted techniques,5,8,32 whereas others suggested that a denser MTA-fill—both in straight and curved-root canals—and better resistance to bacterial penetration is achieved with a combination of hand and ultrasonic techniques.8,33 Though the amount of condensation pressure did not affect the compressive strength, an increase in condensation pressure can interfere with the ingress of water required to hydrate the cement, which in turn may reduce the surface hardness.8,34 Also, irrigation should be performed before the placement, because following placement, irrigation can cause significant washout of the material.
Properties
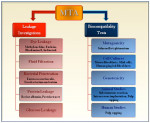
The main characteristic properties of MTA include superior sealing ability, biocompatibility, antimicrobial effect, radiopacity, dimensional stability, and tolerance to moisture over other dental materials such as IRM, amalgam, Ca(OH)2, super EBA, ZOE, etc.5,8-10,13,20,35 Thus, MTA has gained popularity among dental practitioners, especially endodontists, in recent times. Among the above-mentioned properties, the sealing ability and biocompatibility of MTA has been studied extensively9,13 (Figure 1).
Strength
The compressive strength of set MTA is about 70 MPa, which equals IRM and super EBA, but is less than that of amalgam.10,27 Owing to the low compressive strength, placement of MTA in functional areas should be avoided. MTA’s compressive strength is not significantly affected by condensation pressure.8 As discussed earlier, MTA has a prolonged maturation process, with increased compressive strength, push-out strength, and retention strength of the material with time (up to 21 days) in the presence of moisture. The initial compressive strength following 24 hours is 40 MPa, which increases to 67.3 MPa after 21 days. Thus, after 3 weeks, no significant difference in compressive strength was observed between super EBA, IRM, and MTA.8,27 A similar increase in flexure and push-out strength was also observed under moist conditions with the passage of time.36,37 This is because the dicalcium silicate hydration rate is slower than that of tri- calcium silicate.24 Thus, optimal physical properties are gained with time if there is enough moisture following placement at the operation site.5,8,10
Different intracanal irrigants/oxidizing agents can affect the push-out strength/retention strength of GMTA. Use of saline, sterile water, or lidocaine has no effect on the retention strength of the material. However, it is more susceptible to oxidizing agents such as sodium perborate mixed with saline, 30% hydrogen peroxide, and sodium perborate mixed with 30% hydrogen peroxide, whereas 2% chlorhexidine and 5.25% sodium hypochlorite did not significantly affect the strength.38,39 Also, the retention strength of the material is affected by blood-contaminated root surfaces (dentin).40 Investigations also suggested a significant decrease in compressive strength following phosphoric acid (37%) etching. Therefore, restoration with resin-based composite should be postponed for at least 96 hours following placement of MTA.41
Retentive Strength and Bond Strength
The retentive strength of MTA is significantly less than that of glass ionomer or zinc phosphate cement and, thus, it is not considered to be a suitable luting agent.42 Studies have shown that a 4-mm thickness of MTA (apical barrier) offered more resistance to displacement than a 1-mm thickness,43,44 as GMTA-dentin bond strength increases with increases in surface area.8 A total-etch single-bottle adhesive with a resin-based composite or compomer produced higher bond strength than a single-step self-etch system over MTA.45
Microhardness
An exposure to acidic pH (pH5), as observed in inflammatory environment, has an adverse effect on the microhardness of both GMTA and WMTA.29 It is attributed to the absence and growth of needle-like crystals between the cubic crystals during the hydration phase. A 5-mm thickness of MTA is significantly harder than a 2-mm thickness.46 Ethylenediaminetetraacetic acid (EDTA), BioPure MTAD (DENTSPLY Tulsa Dental Specialties), and acid-etching produce surface roughness and significantly reduce the microhardness of MTA.41,47 An increase in condensation pressure results in a more compact mass with fewer micro channels available for water uptake, thus reducing the microhardness of the material.8
pH
Hydrated MTA has an initial pH of 10.2, which rises to 12.5 (similar to calcium hydroxide) 3 hours after mixing and following setting.13,16 The high pH is theorized to be responsible for the antimicrobial action and biological activity of the material. This high pH is attained due to the constant release of calcium from MTA and the formation of Ca(OH)2. The usual pH (11 to 12) of MTA materials decreases slightly with time.48
Sealing Ability (Microleakage)
Results obtained from dye leakage, fluid filtration, protein leakage, and bacterial leakage and endotoxin leakage studies (S. epidermis, S. salivaris, S. marcescens, E. coli, F. nucleatum) indicated that overall MTA showed less microleakage and better sealing ability than traditional materials like amalgam, zinc oxide eugenol-based materials, conventional glass-ionomer, gutta-percha, etc., when used for root-end restoration, root canal obturation, furcation repair, and treatment of immature apices.6,9,13 Expansion of MTA during setting can be responsible for its excellent sealing ability.18,49 Usually a thickness of 3 mm to 5 mm is sufficient to provide a good seal. In the presence of blood contamination, MTA has also been shown to leak significantly less compared to amalgam, IRM, and super EBA.21 No difference in microleakage is reported when used either in an orthograde manner (root-canal filling) or in a retrograde manner (root-end filling).50 However, the presence of residual calcium hydroxide, from the prior placement as an intracanal dressing, can interfere with the adaptation and reduce the sealing ability of MTA. It can act as a mechanical obstacle or can chemically react with MTA.51 In dye leakage investigations, MTA mixed with 10% calcium chloride showed a better sealing ability, with no significant difference in microleakage on addition of chlorhexidine.52,53
Biocompatibility
Biocompatibility studies in general considered both GMTA and WMTA as biocompatible.9,54 No genetic damage, genetic mutation, chromosomal breakage, altered DNA repair capacity, or cellular transformation was observed with MTA. MTA has shown to posses neither mutagenic (Ames mutagenicity assay, Salmonella typhimurium) nor genotoxic effects (single cell gel/comet assay).55,56 Neither freshly mixed nor set MTA displayed neurotoxicity.57 It was found to be less cytotoxic than amalgam, super EBA, and IRM, with set MTA being less cytotoxic than fresh MTA.9,13,54 Enhanced attachment and proliferation of periodontal ligament and gingival fibroblasts were observed on the set-surfaces of MTA.58,59 Similarly, cell cultures studies (animal and human) using human alveolar bone cells, mouse preosteoblasts, osteoblasts, dentinoblasts, and mouse cementoblasts have shown good survival, proliferation, and attachment, with a faster and better growth of cells on the MTA surface.9,35,54 MTA has also shown to have a better stimulating effect on human dental pulp cells than a commercial calcium hydroxide preparation. It was proposed that cellular proliferation is via intra- and extracellular Ca2+ and Erk-dependent pathways, and cell survival is via the Pl3K/Akt signaling pathway.35 Animal cells (rat bone marrow cells, mouse preosteoblasts) and human cells (gingival fibroblasts, periodontal ligament fibroblasts, alveolar bone cells) exposed to MTA have been shown to express alkaline phosphatase, bone sialoprotein, periostin, and osteocalcin, along with the formation of extensive collagenous matrix.60-62 Addition of enamel matrix derivative to MTA has been shown to improve human dental pulp cell differentiation, alkaline phosphatase activity, and mineralization.63 Although addition of chlorhexidine improved the antibacterial properties of MTA, it adversely affected the biocompatibility of the material.9
Animal and human studies have shown minimal or no inflammation to bone and connective tissue following implantation of MTA.9,13 When used (in a canine model) for root-end restoration or for the repair of lateral/furcation perforation, MTA has shown favorable healing characteristics, such as lack of inflammation, no ankylosis, cellular cementum formation (overgrowth), and PDL regeneration between the cementum and alveolar bone.6,9,13 MTA stimulates cytokine release and interleukin production, which may actively promote hard-tissue formation.9,64 Shabahang et al observed that MTA induced hard-tissue formation more often than osteogenic protein-1 and Ca(OH)2.65
Intra-osseous implantation of MTA showed a relatively mild-to-minor inflammatory response, which is more favorable compared to amalgam, super EBA, and IRM.66
Some studies considered that the biocompatibility of MTA is attributable to the release of hydroxyl ions and formation of calcium hydroxide during the hydration process.23,48 Other reports had observed the formation of a white interfacial material (precipitates) between GMTA and tooth structure within 1 to 2 hours when exposed to physiologic fluids (phosphate-buffered physiologic solution) in vivo or with simulated body fluids in vitro.18,20 SEM and x-ray diffraction (XRD) analysis of these precipitates revealed the presence of chemically and structurally similar hydroxyapatite (HA)-like structure with a chemical composition of oxygen, calcium, and phosphorus, along with trace amounts of bismuth, silicon, and aluminum.18 However, the calcium-to-phosphorus ratios reportedly differed from that of natural hydroxyapatite.67 This HA-like structure can release calcium and phosphorus continuously, promoting the regeneration and remineralization of hard tissues and increasing the sealing ability of MTA. The HA-layer also creates a chemical bond between MTA and the dentinal walls.7 The particle size and dimensional shape of MTA can also occlude dentinal tubules, which might harbor microorganisms.68 GMTA has a greater amount of HA-crystal formation than WMTA with the presence of lower levels of silica and phosphorus in GMTA crystals and more calcium ions in WMTA crystals.67
Thus, release of hydroxyl ions, a sustained high pH for extended periods, modulation of cytokine production, formation of calcium hydroxide, and a mineralized interstitial layer (HA) may be responsible for the excellent biocompatibility and biological activity of the material.7,20
Antimicrobial Properties
In vitro studies have shown antibacterial activity of MTA against M. luteus, S. aureus, E. coli, P. aeruginosa, E. faecalis, and S. sanguis.5,8 A study evaluated the antimicrobial property of MTA, amalgam, and super EBA against nine strict anaerobes. MTA was found to have an antibacterial effect on five of the nine facultative bacteria, but no effect on any of the strict anaerobes.69 Thus, the use of MTA as an antibacterial agent may not be very beneficial in endodontic cases. The use of 2% CHX and 0.12% CHX in combination with MTA has been reported to significantly increase the antibacterial effect of both types of MTA.70
Al-Nahazan and Al-Judai71 evaluated the antifungal activity of both freshly mixed and 24-hour-set MTA using a tube dilution test. It was observed that both types were effective against Candida albicans.20 The antifungal effect of MTA might be due to its high pH or to substances that are released from MTA and is dependent on the concentration of MTA; a concentration of 25 mg/mL to 50 mg/mL is required to show an antifungal effect.72
Regenerative Potential and Biological Activity
MTA has the capacity to induce bone, dentin, and cementum formation and regeneration of periapical tissues (periodontal ligament and cementum).7,8,13 MTA provides a good biological seal and can act as a scaffold for the formation and/or regeneration of hard tissue (periapical). It is an osteoconductive, osteoinductive, and cementogenic (cementoconductive and cementoinductive) agent.9,20 MTA stimulates immune cells to release lymphokines and bone coupling factors required for the repair and regeneration of cementum and healing of osseous periapical defects.64,73 MTA can also stimulate periodontal ligament fibroblasts to display osteogenic phenotype and produce osteonectin, osteopontin, and osteonidogen.20,60 Cell culture studies have shown an up-regulation of various cytokines, biological markers, and interlukines, like IL-1α, IL-1β, lL-4, IL-6, osteocalcin, alkaline phosphatase, bone sialoprotein, osteopontin, BMP-2, PGE2, and cyclooxygenase-2, by MTA.9,20 Shabahang et al concluded that MTA can induce the formation of apical hard tissue with significantly greater consistency than osteogenic protein-1 and calcium hydroxide.65 The biologic activity of MTA is attributed to the high pH level associated with formation of calcium hydroxide. Current studies indicated that the biological activity of MTA is attributed to the formation of hydroxyapatite-like precipitate on its surface. GMTA was observed to produce twice as much hydroxyapatite crystals as WMTA, suggesting different levels of bioactivity of the two materials.18,67
Solubility
MTA displays low or nearly no solubility, which is attributable to addition of the bismuth oxide.5,8 Chemical analysis and x-ray diffraction have demonstrated insolubility of 18.8% in water.5 Although MTA forms a porous matrix characterized by internal capillaries and water channels with increased liquid/powder ratio—which can increase the porosity and the solubility further—the solubility levels of GMTA have been shown to be stable over time.13,74
Radiopacity
MTA has a mean radiopacity of 7.17 mm of equivalent thickness of aluminum, which is less than that of IRM, super EBA, amalgam, or gutta-percha.5,75 It has a similar radiodensity to zinc oxide eugenol and slightly greater radiopacity than dentin. Apart from these characteristics, MTA does not react to or interfere with restorative materials like glass-ionomer cements or resin-based composites, which are the commonly used permanent filling materials with MTA.76
Conclusion
An ideal endodontic material should adhere to tooth structure, maintain a good seal, be insoluble in tissue fluids, dimensionally stable and nonresorbable, and radiopaque, and exhibit biocompatibility with a certain degree of bioactivity. Among the various available endodontic materials, MTA is currently the biomaterial that posses most of these characteristics. Nevertheless, the extrapolation of results obtained in in-vitro studies should be undertaken with caution when applied to clinical conditions.
Editor's Note: To read Part 2 of this series, click here.
References
1. Siqueira JF Jr, Rồças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34(11):1291-1301.
2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197-206.
3. Torabinejad M, Pitt Ford TR. Root end filling materials: a review. Endod Dent Traumatol. 1996;12(4):161-178.
4. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19(11):541-544.
5. Rao A, Rao A, Ramya Shenoy R. Mineral trioxide aggregate—a review. J Clin Pediatr Dent. 2009;34(1):1-8.
6. Schwartz RS, Mauger M, Clement DJ, Walker WA 3rd. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999;130(7):967-975.
7. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
8. Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
9. Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-part II: leakage and biocompatibility investigations J Endod. 2010;36(2):190-202.
10. Schmitt D, Lee J, Bogen G. Multifaceted use of ProRoot™ MTA root canal repair material. Pediatr Dent. 2001;23(4):326-330.
11. Oliveira MG, Xavier CB, Demarco FF, et al. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007;18(1):3-7.
12. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white Pro Root MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):809-815.
13. Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24(2):149-164.
14. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40(6):462-470.
15. Camilleri J, Montesin FE, Papaioannou S, et al. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004;37(10):699-704.
16. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349-253.
17. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31(2):101-103.
18. Sarkar NK, Caicedo R, Ritwik P, et al. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31(2):97-100.
19. Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod. 1998;24(11):768-771.
20. Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35(6):777-790.
21. Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: effects of blood contamination. J Endod. 1994;20(4):159-163.
22. Arens D E, Torabinejad M. Repair of furcal perforations with mineral trioxide aggregate: two case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(1):84-88.
23. Camlleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41(5):408-417.
24. Dammaschke T. Gerth HU. Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21(8):731-738.
25. Taylor HFN. Cement Chemistry. 2nd ed. London, England: Thomas Telford, 1997.
26. Ozdemir HO, Ozcelik B, Karabucak B, Cehreli ZC. Calcium ion diffusion from mineral trioxide aggregate through simulated root resorption defects. Dent Traumatol. 2008;24(1):70-73.
27. Torabinejad M, Hong CV, McDonald F, Pitt Ford T R. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349-353.
28. Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives—permanent teeth. J Endod. 2008;34(7):S25-S28.
29. Lee YL, Lee BS, Lin FH, et al. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25(5):787-793.
30. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32(6):569-572.
31. Nandini S, Natanasabapathy V, Shivanna S. Effect of various chemicals as solvents on the dissolution of set white mineral trioxide aggregate: an in vitro study. J Endod. 2010;36(1):135-138.
32. Aminoshariae A, Hartwell GR, Moon PC. Placement of mineral trioxide aggregate using two different techniques. J Endod. 2003;29(10):679-682.
33. Yeung P, Liewehr FR, Moon PC. A quantitative comparison of the fill density of MTA produced by two placement techniques. J Endod. 2006;32(5):456-459.
34. Nekoofar MH, Adusei G, Sheykhrezae MS, et al. The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2007;40(6):453-461.
35. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32(7):601-623.
36. Walker MP, Diliberto A, Lee C. Effect of setting conditions on mineral trioxide aggregate flexural strength. J Endod. 2006;32(4):334-336.
37. Gancedo-Caravia L, Garcia-Barbero E. Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod. 2006;32(9):894-896.
38. Loxley EC, Liewehr FR, Buxton TB, McPherson JC 3rd. The effect of various intracanal oxidizing agents on the push-out strength of various perforation repair materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(4):490-494.
39. Yan P, Peng B, Fan B, et al. The effects of sodium hypochlorite (5.25%), Chlorhexidine (2%), and Glyde File Prep on the bond strength of MTA-dentin. J Endod. 2006;32(1):58-60.
40. Vanderweele RA, Schwartz SA, Beeson TJ. Effect of blood contamination on retention characteristics of MTA when mixed with different liquids. J Endod. 2006;32(5):421-424.
41. Kayahan MB, Nekoofar MH, Kazandağ M, et al. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2009;42(11):1004-1014.
42. Vargas JW, Liewehr FR, Joyce AP, Runner RR. A comparison of the in vitro retentive strength of glass-ionomer cement, zinc-phosphate cement, and mineral trioxide aggregate for the retention of prefabricated posts in bovine incisors. J Endod. 2004;30(11):775-777.
43. Hachmeister DR, Schindler WG, Walker WA 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28(5):386-390.
44. Lawley GR, Schindler WG, Walker WA, Kolodrubetz D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod. 2004;30(3):167-172.
45. Tunç ES, Sönmez IS, Bayrak S, Eğilmez T. The evaluation of bond strength of a composite and a compomer to white mineral trioxide aggregate with two different bonding systems. J Endod. 2008;34(5):603-605.
46. Matt GD, Thorpe JR, Strother JM, McClanahan SB. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one- or two-step apical barrier technique. J Endod. 2004;30(12):876-879.
47. Lee YL, Lin FH, Wang WH, et al. Effects of EDTA on the hydration mechanism of mineral trioxide aggregate. J Dent Res. 2007;86(6):534-538.
48. Duarte MA, Demarchi AC, Yamashita JC, et al. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):345-347.
49. Shipper G, Grossman ES, Botha AJ, Cleaton-Jones PE. Marginal adaptation of mineral trioxide aggregate (MTA) compared with amalgam as a root-end filling material: a low vacuum (LV) versus high vacuum (HV) SEM study. Int Endod J. 2004;37(5):325-336.
50. Andelin WE, Browning DF, Hsu GH, et al. Microleakage of resected MTA. J Endod. 2002;28(8):573-574.
51. Srinivasan V, Waterhouse P, Whitworth J. Mineral trioxide aggregate in paediatric dentistry. Int J Paediatr Dent. 2009;19(1):34-47.
52. Bortoluzzi EA, Broon NJ, Bramante CM, et al. Sealing ability of MTA and radiopaque Portland cement with or without calcium chloride for root-end filling. J Endod. 2006;32(9):897-900.
53. Shahi S, Rahimi S, Yavari HR, et al. Sealing ability of white and gray mineral trioxide aggregate mixed with distilled water and 0.12% chlorhexidine gluconate when used as root-end filling materials. J Endod. 2007;33(12):1429-1432.
54. Perinpanayagam H. Cellular response to mineral trioxide aggregate root-end filling materials. J Calif Dent Assoc. 2009;75(5):369-372.
55. Kettering JD, Torabinejad M. Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995;21(11):537-542.
56. Braz MG, Camargo EA, Salvadori DM, et al. Evaluation of genetic damage in human peripheral lymphocytes exposed to mineral trioxide aggregate and Portland cement. J Oral Rehabil. 2006;33(3):234-239.
57. Asrari M, Lobner D. In vitro neurotoxic evaluation of root-end filling materials. J Endod. 2003;29(11):743-746.
58. Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endod. 2003;30(1):25-29.
59. Pistorius A, Willershausen B, Briseño Marroquin B. Effect of apical root-end filling materials on gingival fibroblasts. Int Endod J. 2003;36(9):610-615.
60. Bonson S, Jeansonne BG, Lallier TE. Root-end filling materials alter fibroblast differentiation. J Dent Res. 2004;83(5):408-413.
61. Tani-Ishii N, Hamada N, Watanabe K et al. Expression of bone extracellular matrix proteins on osteoblast cells in the presence of mineral trioxide. J Endod. 2007;33(7):836-839.
62. Perinpanayagam H. AI-Rabeah E. Osteoblasts interact with MTA surfaces and express Runx2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(4):590-596.
63. Min KS, Yang SH, Kim EC. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod. 2009;35(6):847-851.
64. Koh ET, Torabinejad M, Pitt Ford TR, et al. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37(3):432-439.
65. Shabahang S, Torabinejad M, Boyne PP, et al. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25(1):1-5.
66. Torabinejad M, Pitt Ford TR, Abedi HR, et al. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod. 1998;24(7):468-471.
67. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006;32(5):425-428.
68. Komabayashi T, Spångberg LS. Particle size and shape analysis of MTA finer fractions using Portland cement. J Endod. 2008;34(6):709-711.
69. Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Antibacterial effects of some root end filling materials J Endod. 1995;21(8):403-406.
70. Stowe TJ, Sedgley CM, Stowe B, Fenno JC. The effects of chlorhexidine gluconate (0.12%) on the antimicrobial properties of tooth-colored ProRoot mineral trioxide aggregate. J Endod. 2004;30(6):429-431.
71. Al-Nazhan S, Al-Judai A. Evaluation of antifungal activity of mineral trioxide aggregate. J Endod. 2003;29(12):826-827.
72. Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, et al. Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J Endod. 2005;31(9):684-668.
73. Economides N, Pantelidou O, Kokkas A, Tziafas D. Short-term periradicular tissue response to mineral trioxide aggregate (MTA) as root-end filling material. Int Endod J. 2003;36(1):44-48.
74. Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005; 31(5):376-379.
75. Shah PM, Chong BS, Sidhu SK, Pitt Ford TR. Radiopacity of potential root end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(4):476-479.
76. Nandini S, Ballal S, Kandaswamy D. Influence of glass ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser Raman spectroscopic analysis. J Endod. 2006;33(2):167-172.
About the Authors
Neeraj Malhotra, MDS, PGDHHM
Reader
Department of Conservative Dentistry & Endodontics
KDDC
Mathura, U.P., India
Antara Agarwal, MDS
Senior Dental Consultant
Cosmetic Dentistry & Endodontics
Indus Hygiea, Unit of Indus Speciality Health
Mohali, Punjab, India
Kundabala Mala, MDS
Professor
Department of Conservative Dentistry and Endodontics
Manipal College of Dental Sciences
Mangalore, Karnataka, India