Treatment of Peri-Implantitis with a Diode Laser
A long-term case follow-up
Gregori M. Kurtzman, DDS, MAGD | Markus Weitz, DDS | Ron Kaminer, DDS | Daniel D. Gober, DDS
The prevalence of implant complications is rising significantly as more patients are treated with implants. As with periodontitis associated with natural teeth, periodontal disease can affect implants. This can range from gingival inflammation and the absence of bone loss, to significant bone loss and mobility of the fixture when the disease process is not identified early or a “watch and wait” attitude is taken. Peri-implantitis is a frequent enough occurrence after implant placement that treatment needs to be accomplished to prevent loss of the implant.
Treatment has traditionally involved flapping the site and mechanical debridement with surgical hand instruments to remove any granulation tissue present on the implant’s threads due to the limitations of the surgical tools that might require removal of additional bone to reach areas that are not visible. Success relates to debriding and sterilizing all exposed threads, with success diminishing as more surface area is left untreated. Diode lasers have several benefits related to peri-implantitis treatment. These include easier access to limited access areas due to the small diameter of the flexible glass fiber without the need to remove as much bone as may be required when only surgical instruments are used, and the ability to sterilize the implant’s contaminated surface, eliminating any bacteria that caused the disease and preventing their hampering of healing after treatment. Another added benefit of a diode in these procedures is biostimulation of the mesenchymal stem cells in the surrounding bone and soft tissue, an important tool for regenerative therapy and tissue engineering for better healing.1 Thus, the diode laser is a good adjunct in the treatment of peri-implantitis, improving the clinical results observed with more traditional methods.2
Case Presentation
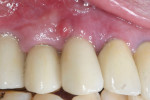
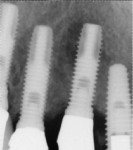
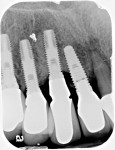
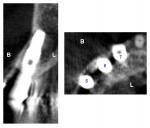
A 64-year-old man presented with a fistula draining on the buccal of the upper right canine. The fistula was located distal to the canine midline in close proximity to the gingival margin (Figure 1). A gutta-percha cone was inserted into the fistula to trace the origination point of the draining infection and a radiograph was taken. Radiographically, it was determined that the fistula traced to the apical of the implant situated at tooth No. 6. Implants had been placed and restored for teeth Nos. 3 through 7 several years prior. The implant was identified as a Branemark Mark III RP (NobelBiocare, www.nobelbiocare.com) at teeth Nos. 4 through 6 and a NobelReplace (NobelBiocare) at tooth No. 7. A radiograph was taken to evaluate the underlying osseous structure around the implant, which demonstrated a radiolucency associated with the apical of implant No. 6 and crestal bone loss with thread exposure under the soft tissue on implant No. 7. Clinically, no recession was noted and no implant mobility was detected.
The patient was informed of the clinical issue and treatment options, including removal of the ailing implant and grafting the site, and following integration of the graft and an appropriate healing period, a new implant could be placed and restored. The other option was to flap the area, clean out any granulation tissue, and treat the site with a diode laser and graft to replace any lost bone. The patient was made aware that the latter option required site evaluation once entered and should the implant have mobility after debridement, the implant area would need to be explanted. The patient chose peri-implantitis repair.
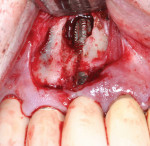
A single dose of prophylactic antibiotic (2 g amoxicillin) was given orally 1 hour preoperatively. A local anesthetic, Articaine (Septocaine® 1:100,000 epinephrine, Septodont, www.septodontusa.com) was administered for local infiltration on the buccal and palatal of the treatment area. A horizontal incision was made from the distal of the first premolar to the mesial of the lateral incisor several millimeters apical to the gingival margin to limit posttreatment recession potential. A vertical releasing incision was made at the mesial and distal extent of the horizontal incision and a full-thickness flap was elevated. Upon flap reflection it was noted that a large dehiscence was present on tooth No. 6 from the crest to several millimeters beyond the apical of this implant. Additionally, some dehiscence was noted on the buccal of implant No. 5 with threads minimally covered with bone over the apical half of the implant, and implant No. 7 presented with 30% to 50% of the threads circumferentially denuded of bone with complete soft-tissue coverage. A hand instrument was used to remove any gross granulation tissue adherent to the bone and exposed implant threads (Figure 3). An activated 300-µm diode tip on the Picasso laser (AMD LASERS, www.amdlasers.com) set at 1.5 W in continuous mode was used to remove any residual granulation tissue on the exposed threads at the defect and sterilize the defect area.3,4 The diode’s fiber tip was placed into physical contact with the implant surface to remove any residual granulation tissue and sterilize the area of any bacteria that contributed to the peri-implantitis, leaving clean threads.
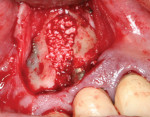
After debridement and sterilization, bleeding points in the osseous walls were created. Bio-Oss® (Geistlich Pharma North America Inc., www.geistlich-na.com), a bovine biocompatible porous bone mineral substitute, was packed into the defect around the implant and allowed to absorb blood from the surrounding tissue to form a coagulated mass. The bone graft was built out buccally to create a new buccal plate covering the entire implant below the crestal level (Figure 4). A piece of resorbable membrane (Ossix Plus®, OraPharma, Inc., www.orapharma.com) was trimmed to overlay the osseous graft and end on native bone, and was placed over the graft under the flap. The flap was repositioned and secured with nine interrupted sutures using 5-0 silk to achieve primary closure. A radiograph was taken to document the bone fill of the osseous graft (Figure 5). Hemostasis was confirmed and the patient dismissed. A prescription for Zithromax was given with the instructions to use as the directed until finished. Additionally, a prescription was given for Dolobid 500 mg for pain to be taken BID for the initial 3 days post-surgically. The patient returned after 1 week for suture removal and indicated no significant postoperative discomfort. The site appeared to be healing normally and he was scheduled for a follow-up. At the next postoperative visit, the site appeared to be healed with a lack of inflammation and the patient was placed on a periodontal recall alternative with his general dentist.
At 5 years post peri-implantitis treatment, a cone-beam computed tomography scan was taken to evaluate the long-term status of the repaired area. The cross-section slice at the right maxillary canine demonstrates the grafted buccal plate has remained at the position completely covering the implant and no sign of further infection is noted (Figure 6).
Discussion
Peri-implantitis can be a challenge to manage. As this case illustrates, bone loss may be progressing for an extended period of time before the clinician becomes aware of it. Treatment requires a surgical approach to remove any granulation tissue that has replaced bone overlaying the implant to achieve any success. The benefit of the diode laser is the fiber can be extended into hard to reach areas around the implant to achieve better sterilization and debridement without the need to remove additional bone for access, which would be necessary if debridement with only surgical hand instruments was used. The diode tip ensures better removal of the granulation tissue and site sterilization to increase treatment success.
Traditional methods have yielded mixed results in removing all of the granulation tissue from the exposed implant threads without altering the implant’s surface. The diode laser has been reported to not cause any visible surface alterations on either polished implant surfaces or coated surfaces. In contrast, when irradiated with the pulsed Er:YAG laser, surface alterations have been reported.5,6 Scanning electron microscope (SEM) analysis demonstrated no damage or alteration of titanium surfaces when in contact with a diode laser, regardless of the power setting. No visible difference between lased and non-lased titanium surfaces after irradiation has been reported, ensuring that the result yields the best surface-guided tissue regeneration compared to either mechanical debridement that can alter the surface by gouging the titanium or coating, or when an Er:YAG laser is used.
Success in peri-implantitis treatment is strongly linked to eliminating bacteria that could hamper regeneration. This becomes more critical with implants that have been surface treated during manufacturing to provide a better surface for integration. These manufacturer-treated implant surfaces yield micro-roughness that bone likes during the initial integration, but also will harbor bacteria when peri-implantitis has occurred. Removal of bacteria in these micro-irregularities is difficult by mechanical means. The diode laser has the ability to decontaminate the exposed surface and threads without any negative effects.7
Once the site has been prepared with the granulation tissue removed and all exposed surfaces decontaminated, osseous grafting is required to ensure the best long-term healing. Without placement of osseous graft material to fill the osseous defects that resulted from the peri-implantitis, the site will most likely not achieve bone fill via organization of a host clot in the void. Membranes are also recommended to allow the body to organize the osseous graft material before soft tissue in-growth can occur from the overlaying flap as soft tissue grows/heals at a much faster rate than hard tissue. The membrane gives the hard tissue an advantage to overcome the soft tissue’s potential to invade the early osseous graft material. Placement of osseous grafting materials and barrier membranes has resulted in greater probing depth reduction and radiographic bone fill than when either material is not used.8
The authors recommend avoiding probing these sites during the healing phase and subsequently after due to the arrangement of connective tissue fibers found around implants. When viewed via SEM, implants have fibers in the gingival aspect where it connects with the implant surface running parallel to the long axis of the implant, which does not provide a physical barrier to the probe, allowing it to push bacteria deeper into the tissue. This may lead to inflammatory changes in the tissue, whereas the fiber orientation around natural teeth is perpendicular to the tooth’s long-axis, providing a physical barrier to the probe.
Conclusion
The key to successful peri-implantitis treatment is early identification to limit bone loss from inflammation and infection. The diode laser is a powerful adjunct to treating peri-implantitis, allowing better access to eliminate more granulation tissue than when only mechanical means are used. It also provides the additional benefits of sterilization of the area and biostimulation to the bone and soft tissue to improve tissue regeneration.
The case illustrated demonstrates that the protocol discussed can provide long-term predictable results, showing 5-year maintenance of the grafted area and an absence of inflammation over that time.
Acknowledgement
Treatment for the case presented performed by Dr. Markus Weitz.
Disclosures
Dr. Gregori Kurtzman has been paid an honorarium by AMD Lasers. Dr. Ron Kaminer is a consultant for AMD Lasers. The other authors have no relevant financial relationships to disclose.
References
1.Barboza CA, Ginani F, Soares DM, et al. Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (Sao Paulo). 2014;12(1):75-81.
2. Lerario F, Roncati M, Gariffo A, et al. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: preliminary clinical study. Lasers Med Sci. 2016;31(1):1-6.
3. Roncati M, Lucchese A, Carinci F. Non-surgical treatment of peri-implantitis with the adjunctive use of an 810-nm diode laser. J Indian Soc Periodontol. 2013;17(6):812-815.
4. Mettraux GR, Sculean A, Bürgin WB, Salvi GE. Two-year clinical outcomes following non-surgical mechanical therapy of peri-implantitis with adjunctive diode laser application. Clin Oral Implants Res. 2016; 27(7):845-849.
5. Stubinger S, Etter C, Miskiewicz M,et al. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er:YAG, carbon dioxide, and diode laser irradiation. Int J Oral Maxillofac Implants. 2010;25(1):104-111.
6. Castro GL, Gallas M, Núñez IR, et al. Scanning electron microscopic analysis of diode laser-treated titanium implant surfaces. Photomed Laser Surg. 2007;25 (2):124-128.
7. Preissner S, Wirtz HC, Tietz AK, et al. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: an in-vitro-study. J Biophotonics. 2016;9(6):637-644.
8. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014; 85(8):1027-1041.
About the Author
Gregori M. Kurtzman, DDS, MAGD
Private Practice
Silver Spring, Maryland
Markus L. Weitz, DDS
Private Practice
Cedarhurst, New York
Ron Kaminer, DDS
Private Practice
Hewlett and Oceanside, New York
Daniel D. Gober, DDS
Private Practice
Cedarhurst, New York