The Evolution of Implantology
How science and clinical practice are improving patient outcomes.
By Barry P. Levin, DMD
No area of dentistry evolves more rapidly than implantology. From placement techniques, to surface topography, to loading protocols and imaging, the field of dental implantology is as dynamic as it is rewarding. This article aims to point out several of the most important emerging technologies available to clinicians today. It is far from an exhaustive review, merely an attempt to describe some of the more impactful developments in recent times.
The largest paradigm shift from a “placement” perspective is the advent of guided-bone regeneration (GBR). At its inception, implantology was a “surgically driven” modality. Dedicated surgeons collaborated with prosthodontists to plan edentulous cases for rehabilitation. The aspect of treatment planning these patients that led to many shortcomings was the predictable bone resorption that followed tooth loss. Recent investigators demonstrated rapid dimensional changes of the alveolar bone after extractions.1 Panoramic study was coupled with diagnostic mock-ups and radiopaque markers to select the most favorable anatomic locations for implant insertions. Often the prosthetic reconstruction was “built up” from the surgically driven placement of implants. These restorations were often not without complications. Due to less than ideal biomechanical connections of “stacked” components—such as implant–abutment, abutment–abutment, and abutment–prosthesis connections—the fracture of screws, prostheses, and implant fixtures was not uncommon.
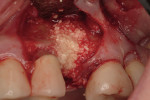
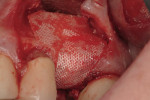
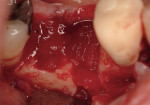
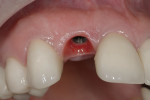
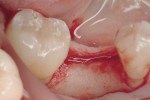
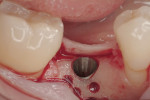
Regeneration of alveolar bone is a reality. This possibility has facilitated more mechanically favorable implant positioning, and driven the evolution from surgically to restoratively driven placements. The minimization of oblique and lateral forces on fixtures and components made possible by regenerative surgery is closely related to the dramatic decrease in mechanical component failures and preservation of crestal bone in the long term. In the last decade, surgeons have aimed to provide favorable anatomic conditions for implant positioning, starting at the time of extraction. This has resulted in two specific treatment courses, the first being immediate implant placement, ie, placing the implant body directly into the extraction socket (Figure 1). What was once thought of as a modality that prevented physiologic socket modeling by immediate placement has been disproven.2,3 Immediate placement does allow the surgeon the opportunity to shorten overall treatment time, and exploit normal osseous “fill” around an implant inserted into an extraction site. The second option is taking measures to prevent horizontal and vertical bone modeling prior to implant placement at a future time. This option may be further divided into delayed implant placement or “socket preservation” where bone augmentation is performed at the time of extraction (Figure 2, Figure 3 and Figure 4). In delayed placement, time is allowed for soft tissue healing, but not appreciable osseous fill of the extraction socket. This usually results in implant insertion 6 to 8 weeks after tooth removal. Some investigators4 advocate this technique as the preferred manner of anterior tooth replacement. With socket preservation, a bone-replacement graft and a membrane are placed to preserve alveolar ridge dimension favoring prosthetically driven implant placement.5,6
Original techniques for GBR incorporated tissue-exclusive barrier membranes. These ePTFE membranes were adapted over space-maintaining or partially space-maintaining alveolar defects. When the collapse of these barriers was not avoidable, bone-graft materials were placed into the physical space between the defect walls and the overlying membrane. The ideal graft material would be osteogenic; viable cells capable of surviving the transplantation of autogenous bone from the remote donor site to the area of desired regeneration. The limitations of these procedures include morbidity associated from harvesting autogenous bone from both intra- and extraoral sources, limited amounts of intraoral bone necessary for grafting larger defects, and the issue of graft resorption, especially when autogenous block grafts are used. These are merely a few shortcomings of autogenous bone, and have resulted in the common use of bone substitutes, such as bone allograft, xenograft, and alloplasts. The major advantages of these solutions are the elimination of a donor site required for autogenous bone grafting and the limitless supply of a uniform graft material.
Another evolution of GBR technology is the advent of resorbable barrier membranes. Investigators have reported high incidences of premature membrane exposure and the compromised or failed outcomes when this occurs. The non-resorbable barriers also require removal. Both synthetic and animal-derived (mainly bovine and porcine collagen) materials will degrade, eliminating the need for their removal. Techniques such as cross-linking of collagen fibrils extend resorption time, sustaining cell-exclusionary function.7 Another advantage of these biomaterials is easier management of early exposures due to the natural degradation of the barriers.
One of the most important evolutions of bone regeneration is the introduction of biologic mediators. Several recombinant proteins or “growth factors” are capable of driving and accelerating the regeneration of alveolar bone. The most intensely investigated of these growth factors are the bone-morphogenetic proteins, in particular BMP-2. Unlike other commercially available “biologics,” rhBMP-2 is an “osteoinductive” protein, capable of differentiation. This implies that when bound by the protein, BMP-2 receptors found on undifferentiated stem cells of mesenchymal origin morphologically follow the osteoblastic pathway of development. By manipulating the wound-healing process, clinicians can drive the regenerative process on a cellular level, toward the restoration of deficient bone (Figure 5, Figure 6 and Figure 7). In a multicenter study, Fiorellini et al8 demonstrated the clinical efficacy of grafting anterior extraction sockets with buccal wall deficiencies resulting in favorable implant receptor sites. In an animal study, Jovanovic et al9 showed that implants placed into bone regenerated with rhBMP-2 can be favorably loaded for 12 months. This biologic mediator has already proven to be clinically effective for use in sinus grafting and long-term loading in multicenter clinical trials.10,11 This may be most important for medically compromised patients who may not respond to traditional therapy due to wound-healing impairments related to medications, systemic diseases, or substantial defects that would not heal predictably.12
These are but a few of the many evolutions in surgical therapy that have led to enhanced outcomes related to implant placements. Another evolution in implant therapy is the rapid development of digital radiography. From a real-time point of view, digital x-rays in the surgical operatory afford surgeons the practical opportunity to take intraoperative radiographs. This not only reduces radiation exposure doses, but also shortens surgery time because the time necessary for developing x-ray films is eliminated. Shorter surgeries reduce morbidity associated with prolonged procedures.
The introduction of cone-beam, computer tomography (CBCT) into the private-practice setting may be the most impactful development in implantology in the last decade. CBCT not only results in lower radiation exposures compared to spiral and conventional CT scans, but increases case acceptance because patients do not have to present to a remote radiologic center for image acquisition. Also, because these scanners are office-based, costs can be reduced, again leading to better patient acceptance.
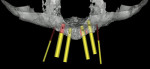
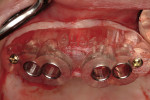
Along with the opportunity to appreciate vital anatomic structures,13,14 digital 3-dimensional imaging allows the surgeon to work with one of several planning software programs. These programs aid in treatment planning, implant dimensional selection, and the generation of surgical guides.15-18 Computer-guided implant surgery gives surgeons and patients the reassurance of safe and accurate implant placement (Figure 8 and Figure 9). It also can be implemented in appropriate situations to provide minimally invasive or “flapless” implant insertions. When adequate hard and soft tissue conditions exist, prosthetically driven implant placements can be performed without the elevation of mucoperiosteal flaps (Figure 10 and Figure 11). Several investigators concluded that this might result in shorter procedure times, less postoperative swelling and pain, shorter recovery times, and better case acceptance. It should be pointed out that these types of procedures do not allow for hard and/or soft tissue augmentations simultaneous with implant placement.
The reduction in overall treatment time cannot be overlooked in the evolution of implant dentistry. What was considered standard time for osseointegration at implantology’s inception (3 to 4 months in the mandible and 6 to 8 months in the maxilla) caused many patients and clinicians to consider alternative tooth-replacement options. As micro- and macroscopic implant topography has evolved, the rate of osseointegration has significantly shortened. Often the overall treatment time of standard implant therapy compares favorably to alternative techniques, such as the fabrication of fixed partial dentures. This facet alone has caused many patients and clinicians to view implantololgy as a viable option.
Modern surface technology is proven to accelerate bone apposition, and chemical modifications of macro and micro-roughened titanium surfaces have hastened osseointegration time even further.19,20 The obvious advantage to rapid osseointegration is shortening implant treatment time, comparable to that of more conventional, fixed restorative therapy. Morton et al21 showed a nearly 98% success rate of implants with a chemically modified surface loaded after 21 days of non-submerged healing after 2 years of follow-up. Another exploitation of these “osteophylic” implant surfaces is their use in immediate temporization and immediate functional loading. In a case series of 30 consecutively temporized implants in the esthetic zone where immediately placed implants were provided with non-functional provisional restorations at the time of surgery, Levin22 demonstrated an 100% implant-survival rate after an average loading time of 9 months. A multicenter study demonstrated that implants placed and immediately loaded demonstrated comparable survival to the same implants loaded at 4 to 5 weeks in posterior sites.23 In a canine study, Berglundh et al24 demonstrated more rapid osseointegration for implants chemically modified with fluoride, compared to implants otherwise identical in terms of macro and micro-topography. In a human trial, Cooper et al25 demonstrated that fluoride-modified titanium implants used in immediate-temporization situations elicited favorable bone and soft tissue responses, regardless of whether the implants were inserted into extraction sites or healed ridges. This chemically (fluoride)-modified titanium surface has been shown to stimulate the proliferation and extracellular matrix synthesis of bone-marrow–derived, mesenchymal stem cells.26 Histologically, this surface has demonstrated more rapid bone-to-implant contact compared to the same titanium-oxide-blasted surface without fluoride treatment.24
Not only are immediately delivered temporaries attractive to patients for the purpose of negating the need for removable provisional restorations, but it affords the clinician to opportunity to develop soft tissue contours prior to initiating definitive restorative therapy (Figure 12, Figure 13 and Figure 14). Chee et al27 theorized over a decade ago that these temporary restorations allow for the earliest opportunity to maintain existing soft-tissue morphology. Stein and Nevins28 discussed the relationship between provisional crowns and implant positioning as the “guided gingival frame” and how placement depth can affect the level of clinician’s control of developing esthetic gingival contours. With the loss of the tooth in the absence of a coronal restoration, the papilla lacking support leads to a flattened area, which in turn leads to difficulty in reestablishing the lost papilla when the final restoration is placed. Immediate provisionalization allows for support and preservation of the papilla until a final restoration is placed.
Of great interest and debate in recent years is the effect on peri-implant tissues that the fixture-abutment connection plays. Original prosthetic connections were that of a “butt joint” where the diameter of the fixture head was identical to that of the portion of the abutment that connected to the implant. These implants were considered “successful” with a 1.5-mm to 2-mm level of radiographic bone loss after 1 year of loading.29,30 Various theories pertaining to the cause of this bone loss have been espoused; however, the most consistently discussed concepts are that an inflammatory infiltrate will persist around this junction or “micro-gap.”31 Another reason for this type of bone modeling at this junction is the necessity for the “biologic width” to form around endosseous implants in a manner analogous to that found around natural teeth.32 Tarnow et al33 demonstrated the 3-dimensional nature of this phenomenon in a radiographic study looking at “butt-joint” type implants. What this group found is that the horizontal component of the peri-implant biologic width is approximately 1.5 mm. With two adjacent implants, the necessity to space the fixtures at least 3 mm apart is crucial in preserving the inter-implant “peak” of bone. This may be related to the presence or absence of a proximal soft tissue papilla. This amount of bone loss could be related to catastrophic soft tissue recession in esthetically critical sites. Patients with thin biotypes are more susceptible to soft tissue recession, and implant esthetics are more challenging.34
Most implant manufacturers have addressed this potential shortcoming of butt-joint connections by capitalizing on incidental findings of researchers who used “mismatched” abutments, which were of smaller diameters than the heads of the restored implants. This concept became known as “platform switching.” The encouraging results reported by many clinicians is the minimal vertical bone loss found radiographically compared to older designs. Canullo et al35 found an inverse relationship between the amount of abutment–implant mismatching and the extent of marginal bone loss. The bone preservation around the top of the implants has been suspected of leading to better soft-tissue maintenance, correlating to more pleasing esthetic outcomes. In radiographic and histologic studies, Jung and Cochran36 found limited vertical bone loss in the canine model when evaluating mismatched implant–abutment situations. This may also change the way treatment planning is performed when adjacent implants are indicated but previously avoided, to prevent loss of proximal hard and soft tissues. In a retrospective study of adjacent platform-switched implants, Rodriguez-Ciurana et al37 demonstrated that the mean inter-implant distance was less than 3 mm and it was possible to maintain the proximal bone peak over 60% of these situations.
Other geometric modifications have come about in attempts to preserve marginal bone levels. Varying the height of the implant top as it related to the proximal and radicular (buccal and palatal) surfaces has been experimented with in recent years. Scalloped implants and beveled implants are currently marketed. Scalloped implants have reported mixed results. In a clinical trial evaluating non-scalloped and scalloped implants, the scalloped implant was found to be associated with significantly more marginal bone loss.38 A beveled implant, indicated for placement in “sloped” ridges, is intended to prevent overseating the implant to the level of the buccal crest at the expense of proximal bone height (Figure 15 and Figure 16). These implants are relatively new; however, early clinical research is rather promising.8
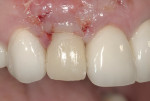
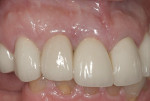
The restorative side of implant dentistry is as dynamic as its surgical counterpart. Attempts to improve not only the strength of restorative materials, but also esthetic efficacy and convenience in fabrication, are countless. The most exciting event in this evolution is the realization of CAD/CAM technology being implemented into clinical practice. This type of technology includes, but is not limited to, digital impression taking, milling rather than casting restorations, and the use of all-ceramic restorations of comparable strength to metallic materials. Glauser et al39 found excellent restoration survival rates with zirconia abutments for implant-supported restorations at 4 years. Sailer et al40 concluded that for patients with thin peri-implant soft tissues, the method of choice for restoration in esthetically demanding situations should be zirconia abutments and all-ceramic crowns. The confidence of acceptable fatigue resistance of porcelain bonded to zirconia abutments is now a realization.41 This has allowed restorative dentists to provide “metal-free” restorations, optimizing the esthetic potential of therapy, especially in patients with thin biotypes, where metallic grey shadows showing through the very friable and thin tissues may seriously compromise outcomes of treatment (Figure 17, Figure 18 and Figure 19).
Conclusion
As presented in this article, there is no shortage or stoppage of science pushing implant dentistry forward. Although manufacturers are funding significant amounts of research, the basis for the standard of care should always remain in objective science. Techniques that make procedures seem “easy” and applicable for the novice should be scrutinized for scientific evidence supporting their use. With discretion, the progressive clinician will find many emerging techniques and materials available to simplify and elevate the level of patient care.
This is a very exciting time for implant dentistry. Regeneration at the molecular level is restoring lost or damaged tissues. Dental materials are closer than ever to mimicking hard dental structure. The goal of tooth replacement equivalent to natural dentition is not a reality yet, but closer than it has ever been.
References
1. Araújo MG, Lindhe J. Ridge alterations following tooth extraction with and without flap elevation: an experimental study in the dog. Clin Oral Impl Res. 2009;20(6):545-549.
2. Caneva M, Botticelli D, Salata LA, et al. Flap vs “flapless” surgical approach at immediate implants: a histomorphometric study in dogs. Clin Oral Impl Res. 2010;21:1314-1319.
3. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820-828.
4. Buser D, Halbritter S, Hart C, et al. Early implant placement with simultaneous guided bone regeneration following single-tooth extraction in the esthetic zone: 12-month results of a prospective study with 20 consecutive patients. J Periodontol. 2009;80(1):152-162.
5. John V, De Poi R, Blanchard S. Socket preservation as a precursor of future implant placement: review of the literature and case reports. Compend Contin Dent Educ. 2007;28(12):646-654.
6. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with a freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;990-999.
7. Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005;16(2):210-219.
8. Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605-613.
9. Jovanovic SA, Hunt D, Benard GW, et al. Long-term functional loading of dental implants in rhBMP-2 induced bone. A histologic study in the canine ridge augmenation model. Clin Oral Impl Res. 2003;14:793-803.
10. Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693-1707.
11. Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67:1947-1960.
12. Levin BP. Minimizing risks for patients taking bisphosphonates. Implant Dentistry Today. U.K. March 2011;
13. Jacobs R, Mraiwa N, vanSteenberghe D, et al. Appearance, location, course, and morphology of the mandibular incisive canal: an assessment on spiral CT scan. Dentomaxillofacial Radiology. 2002;31;322-327.
14. Quirynen M, Mraiwa N, vanSteenbrghe D, Jacobs R. Morphology and dimenstions of the mandibular jaw bone in the interforaminal region in patients requiring implants in the distal areas. Clin Oral Impl Res. 2003;14:280-285.
15. Pettersson A, Kero T, Gillot L, et al. Accuracy of CAD/CAM-guided surgical template implant surgery on human cadavers: part 1. J Prosthet Dent. 2010;103:334-342.
16. Horwitz J, Zuabi O, Machtei EE. Accuracy of a computerized tomography-guided template-assited placement system: an in vitro study. Clin Oral Impl Res. 2009;20:1156-1163.
17. Katsoulis J, Pazera P, Mericske-Stern R. Prosthetically driven, computer-guided implant planning for the edentulous maxilla: a model study. Clin Implant Dent Related Res. 2009;11:238-245.
18. Dreiseidler T, Beugebauer J, Ritter L, et al. Acccuracy of a newly developed integrated system for dental implant planning. Clin Oral Impl Res. 2009;20:1191-1199.
19. Bornstein MM, Valderama, P, Jones A, et al. Bone apposition around 2 different sla titanium surfaces: a histomorphometric study in canine mandibles. Clin Oral Impl Res. 2008;19:233-241.
20. Schwarz F, Heren M, Wieland M, et al. J Clin Periodontol. 2007;34:78-86.
21. Morton D, Bornstein MM, Wittenben J-G, et al. Early loading after 21 days of healing of a nonsubmerged titanium implants with a chemically modified sla surface: 2-year results of a prospective two-center study. Clin Oral Impl Res. 2010;12:9-17.
22. Levin BP. Immediate temporization of immediate implants in the esthetic zone: evaluating survival and bone maintenance. Compend Cont Educ Dent. 2011;32:52-62.
23. Zollner A, Ganeles J, Korostoff J, et al. Immediate and early non-occlusal loading of Straumann implants with a chemically modified surface (SLAactive) in the posterior mandible and maxilla: interim results from a prospective multicenter randomized-controlled study. Clin Oral Impl Res. 2008;19:442-450.
24. Berglundh T, Abrahamsson, I, Albouy J-P, Lindhe J. Bone healing at implants with a fluoride-modified surface: an experimental study in dogs. Clin Oral Impl Res. 2007;18:147-152.
25. Cooper LF, Raes F, Reside GJ, et al. Comparison of radiographic and clinical outcomes of single-tooth dental implants placed in healed alveolar ridges and extraction sockets. Int J Oral Maxillofac Implants. 2010;25:1222-1232.
26. Guida L, Annunziata M, Rocci A, et al. Biological response of human bone marrow mesenchymal stem cells to fluoride-modified titanium surfaces. Clin Oral Impl Res. 2010;21:1234-1241.
27. Chee WW, Donovan T. Use of provisional restorations to enhance soft-tissue contours for implant restorations. Compend Cont Educ Dent. 1998;19:481-489.
28. Stein JM, Nevins M. The relationship of the guided gingival frame to the provisional crown for a single-implant restoration. Compend Cont Educ Dent. 1996;17(12):1175-1182.
29. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1985;1(1):11-25.
30. Jemt T, Lekholm U. Single implants and buccal bone grafts in the anterior maxilla: measurements of buccal crestal contours in a 6-year prospective clinical study. Clin Implant Dent Relat Res. 2005;7(3):127-135.
31. Broggini N, McManus LM, Hermann JS, et al. Persistant acute inflammation at the implant-abutment interface. J Dent Res. 2003;82(3):232-237.
32. Vignoletti F, de Sanctis M, Berglundh T, et al. Early healing of implants placed into fresh extraction sockets: an experimental study in the beagle dog III: soft tissue findings. J Clin Periodontol. 2009;36:1059-1066.
33. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol. 2000;71:546-549.
34. Kan JY, Rungcharassaeng K, Umezu K, Kois JC. Dimensions of peri-implant mucosa: An evaluation of maxillary anterior single implants in humans. J Periodontol. 2003;74:557-562.
35. Canullo L, Rossi Fedel G, Iannello G, Jepsen S. Platform switching and marginal bone-level alterations: the results of a randomized-controlled trial. Clin Oral Impl Res. 2010;21:115-121.
36. Jung RE, Jones AA, Higginbottom FL, et al. The Influence of non-matching implant and abutment diameters on radiographic crestal bone levels in dogs. J Periodontol. 2008;79(2):260-270.
37. Rodriguez-Ciurana X, Vela-Nebot X, Segala-Torres M, et al. The effect of interimplant distance on the height of the interimplant bone crest when using platform-switched implants. Int J Periodontics Restorative Dent. 2009;29:141-151.
38. Tymstra N, Raghoebar GM, Vissink A, et al. Treatment outcome of two adjacent implant crowns with different implant platform designs in the aesthetic zone: a 1-year randomized clinical trial. J Clin Periodontol. 2011;38:74-85.
39. Glauser R, Sailer I, Wohlwend A, et al. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int J Prosthodont. 2004;17:285-290.
40. Sailer I, Zembic A, Jung Re, et al. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007;2(3):296-310.
41. Magne P, Gandolfi-Paranhos MP, Burnett Jr. LH, et al. Fatigue resistance and failure mode of novel-design anterior single-tooth implant restorations: influence of material selection for type III veneers bonded to zirconia abutments. Clin Oral Impl Res. 2011;22:195-2000.
About the Author
Barry P. Levin, DMD
Clinical Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania



 www.guidor.com
www.guidor.com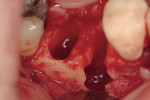
 www.medtronic.com
www.medtronic.com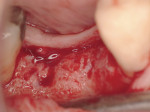
 www.materialise.com
www.materialise.com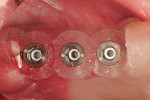 www.astratechdental.us
www.astratechdental.us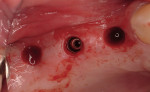
 www.astratechdental.com
www.astratechdental.com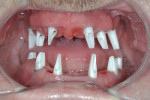 www.straumann.us
www.straumann.us