Tooth Whitening With a Novel Emulsion Technology
Tiezhou Hou, PhD; Hongyan Zheng, MA; Nataliya Gurich, PhD; Mary Kay Anastasia, BA; and Jiahui Li, BS
ABSTRACT
Objective: To compare the effect on tooth color of a hydrogen peroxide whitening emulsion to that of a whitening dentifrice. Methods: In this 21-day, randomized, two-treatment, parallel-group clinical study, 70 healthy adults with six gradable, natural maxillary anterior teeth were randomly assigned to use a whitening, peroxide-free dentifrice (control group) or a combination of a regular dentifrice and a 2.5% hydrogen peroxide leave-on whitening emulsion (test group). All participants were instructed to brush twice daily with an assigned manual toothbrush and dentifrice; participants in the test group were also instructed to apply the emulsion twice daily. High-resolution digital imaging was used to determine color values (b* and L*) for six maxillary anterior teeth at baseline and after 14, 28, and 42 applications (7, 14, and 21 treatment days, respectively). Tolerability and safety were evaluated via oral status interview and examination. Results: After 14 applications, the test group demonstrated a statistically significant reduction in yellowness (b*) and increase in lightness (L*) relative to baseline and to the control group (P < .001 for all). Significantly greater improvements in both color parameters were observed for the test group versus the control group for all timepoints in the study. Both treatments were well tolerated. Conclusion: The whitening emulsion delivered statistically significantly better tooth whitening efficacy relative to a whitening dentifrice and was well tolerated. These results confirm that 2.5% hydrogen peroxide leave-on whitening emulsion is an effective and safe choice for tooth whitening.
Tooth color is a significant contributor to overall dental self-image.1,2 Yet studies suggest that more than 50% of people are dissatisfied with their tooth color.1,3,4 Tooth whitening is a popular and effective solution to this problem,5 with research showing whiter teeth confer greater self-confidence and improve quality of life.1,4, 6-9
There are two components to tooth whitening: removal of stains on the tooth surface and bleaching chromogens below the tooth surface. The most common dental bleaching agent is hydrogen peroxide, which is either incorporated directly into a bleaching product or generated from its precursor (carbamide peroxide) when the product is applied to the teeth.10-12 Modes of delivery include in-office gel,13,14 professionally administered at-home trays for daytime or nighttime wear,15-17 and over-the-counter applications such as rinses, dentifrices, paint-on gels, tray-delivered gels, and gel-coated strips.18 Regardless of delivery method, hydrogen peroxidebleaches stain by producing free radicals that break the double bonds of organic stain-causing molecules, changing the way these molecules reflect light and producing a lighter, whiter tooth color.10,19 Whitening efficacy is dependent on the concentration of hydrogenperoxide and sufficient contact time with the tooth surface.20 The process is a mass balance function where hydrogen peroxide concentration at the chromogens may be driven by diffusion time, hydrogen peroxide formulation, or hydrogen peroxide application site and/or methodology.
The whitening emulsion investigated in this study represents new technology consisting of microdroplets of an aqueous hydrogen peroxidesolution, suspended in a petrolatum gel. A unique aspect of this formulation is the base prevents dehydration, one of the primary contributors to bleaching-related sensitivity.21 When the emulsion is applied to teeth, the hydrophilic microdroplets migrate out of the base to the hydrophilic tooth surface. The hydrophobic base then serves as a barrier, preventing saliva from rinsing away the peroxide and therefore extending contact time. The purpose of this study was to compare the effect of this 2.5% hydrogen peroxide whitening emulsion on tooth color to the effect of a marketed whitening dentifrice during 21 days of use.
Methods
This 21-day, single-center, randomized, two-treatment, parallel-group, examiner-blind study was conducted at the Beijing Sino German Union Cosmetic Institute Co., Ltd., Beijing, P.R. China. Institutional review and approval of the protocol was obtained by Beijing Sino German Union Cosmetic Institute Co., Ltd., Beijing, P.R. China. The study was conducted in compliance with the International Conference on Harmonization's Good Clinical Practice Consolidated Guidelines. All participants provided written informed consent.
Participants
Enrolled participants were in good general health and had six gradable, natural maxillary anterior teeth with a VITA® shade score of A2 or darker. Exclusion criteria included severe or atypical intrinsic staining such as that caused by tetracycline, fluorosis, or hypocalcification; self-reported tooth sensitivity or sensitivity to hydrogen peroxide; periodontitis; active treatment for gingivitis, periodontitis, or caries; presence of fixed orthodontic devices on the facial surfaces of the anterior teeth; presence of malocclusion that would impact treatment; use of tobacco, e-cigarettes, or vaping devices; current or anticipated pregnancy; or any condition or disease that could be expected to interfere with examination procedures or with the participant's safe completion of the study. Qualified participants agreed not to partake in any other oral care study or have any elective dentistry performed during the study period.
Clinical Procedures and Study Products
At visit 1, all participants provided written informed consent, medical history, and demographic information, and inclusion and exclusion criteria were evaluated and documented. Participants were provided with regular 0.243% sodium fluoride dentifrice (Crest® Cavity Protection, The Procter & Gamble Company, dentalcare.com) and a soft, flat-trim manual toothbrush (Crest Velvet Superthin toothbrush OM169, The Procter & Gamble Company) to use in place of their daily hygiene products for the duration of the study.
At the baseline visit (visit 2), participants received an oral examination and an oral status interview (OSI), and digital imaging was performed on the facial surfaces of anterior teeth. Throughout the study, oral examiners and digital imaging system operators were blind to each participant's treatment assignment. Digital imaging analysis was used to describe key components of the 3-dimensional coordinate space: L* (white-black), a* (red-green), and b* (yellow-blue).22
Participants were randomly assigned to one of two treatment groups using an encoded balance and assignment program supplied by the sponsor: (1) test group: 2.5% hydrogen peroxide whitening emulsion (Crest® Whitening Emulsions, The Procter & Gamble Company), an applicator, 0.243% sodium fluoride dentifrice (Crest Cavity Protection), a soft, flat-trim manual brush (Crest Velvet Superthin toothbrush), and a timer; or (2) control group: 0.243% sodium fluoride whitening dentifrice (Crest® Dual Action 3D White, The Procter & Gamble Company) a soft, flat-trim manual brush (Crest Velvet Superthin toothbrush) and a timer. As part of the randomization, participants were stratified according to age (≤40 vs >40), maxillary L* (≤72.5 vs >72.5), and maxillary b* (≤15.8 vs >15.8).
All participants were instructed to brush twice daily in their customary manner, morning and evening, with their assigned brush and dentifrice. Participants in the test group were instructed to apply the emulsion to the facial surfaces of the anterior maxillary teeth twice daily, morning and evening, and to refrain from eating, drinking, or chewing gum for 30 minutes after applying the emulsion.
At designated timepoints continuance criteria were assessed, OSIs were conducted, and oral examinations and digital imaging were performed. Participants in the test group reported to the testing site each morning, and emulsion application was observed by staff. On assessment days, digital photographic images were taken before the emulsion was applied.
Scoring Procedures
Digital photographic imaging was performed with a high-resolution digital camera. Two 150-watt lights located on each side of the camera provided lighting. A connected personal computer recorded the images for later analyses. The system was calibrated daily prior to use and a color standard was centered and imaged every half hour.
Images were collected according to the method described previously, and color values (L*, a*, and b*) were generated for the facial surfaces of the six maxillary anterior teeth using validated computer-aided masking and analysis.22
Statistical Analysis
Thirty-five participants were assigned to each of the two treatment groups (N = 70). This satisfied the sample size of 30 participants per group to ensure 80% power to detect a difference of 0.5 b* units between treatment groups with a standard deviation (SD) of 0.68. Power analyses were conducted with a two-sided test and an α level of .05.
The primary endpoint of this study was the change in b* after 42 applications (representing 21 days of twice a day treatment) relative to baseline. Secondary endpoints were changes in b* assessed after 14 and 28 applications (7 and 14 treatment days, respectively) and changes in L*. The tested null hypotheses were that the mean level of color change from baseline was equal to zero for each treatment group, and that the mean level of color change from baseline was equal between treatments. The alternative hypotheses were that color change was not equal to zero and was not equal between treatments.
Demographic data, digital color measurements, and safety data were summarized. Mean comparisons to baseline were performed with paired difference t tests. The analysis of covariance method was used to compare treatment groups with the efficacy endpoint as the response and baseline and age as covariates. Unequal variances were modeled for each treatment. All statistical tests were two-sided with a 5% level of significance.
Results
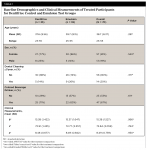
From among77 participants screened, 70 were enrolled and randomized to treatment (57 females and 13 males) with a mean age (SD) of 38.7 (8.37) years. Sixty-eight participants completed the study. (Two subjects, one in each group, voluntarily withdrew from the trial.) At baseline, mean b*, L*, and a* did not differ significantly between groups (P≥ .784). There was no significant between-group difference in any baseline demographic characteristics (P ≥ 0.281). See Table 1.
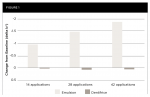
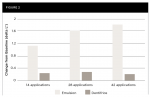
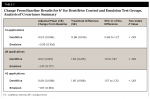
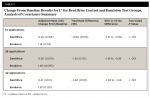
After 14 applications (7 treatment days) and continuing throughout the study, the test group exhibited a statistically significant mean reduction in yellowness (Δb*) relative to baseline (P < .001). After 42 applications (21 treatment days), the mean (SD) change from baseline for the test group was −1.85 (0.899) (P < .001). The control group showed no significant improvement in b* at any timepoint. See Figure 1. The between-group difference in adjusted mean Δb* relative to baseline (standard error [SE]) was 0.98 (0.145) after 14 applications (P < .001). By 42 applications, the between-group difference (SE) was 1.95 (0.188) (P < .001) (Table 2).
After 14 applications and continuing throughout the study, the test group exhibited a statistically significant mean increase in lightness (ΔL*) relative to baseline (P < .001). After 42 applications, the mean (SD) changes from baseline for the test and control group were 1.79 (0.767) (P< .001) and 0.23 (0.536) (P = .024), respectively. See Figure 2. The between-group difference in adjusted mean ΔL* relative to baseline (SE) was −0.90 (0.155) after 14 applications (P < .001). By 42 applications, the between-group difference (SE) was −1.60 (0.157) (P < .001) (Table 3).
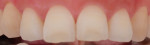
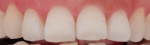
Figure 3 and Figure 4 show clinical images for a participant in the test group at baseline (Figure 3) and after 42 applications (Figure 4) of the emulsion product.
No oral soft-tissue irritation attributed to treatment was reported. Two participants (5.7%) in the test group reported mild tooth sensitivity categorized as possibly related to treatment. In both instances, the tooth sensitivity was described as "not bothersome" and lasted less than 5 minutes. No participants withdrew from the study due to treatment-related adverse events.
Discussion
This study demonstrated that a 2.5% hydrogen peroxide whitening emulsion produced a statistically significant reduction in yellowness and increase in lightness relative to baseline and to that produced by a whitening dentifrice. Color improvements were observed after 14 applications (7 treatment days), and improvements continued through 42 applications. Only two instances of mild tooth sensitivity occurred, each lasting for less than 5 minutes.
Similar to traditional tooth-bleaching products, the novel emulsion technology contains hydrogen peroxideto eliminate stains both on and within tooth enamel.19,23 Separate research by Suszcynsky-Meister et al confirms the bleaching effect of the emulsion is safe to hard tissues.24 Simulated overuse conditions evaluating microhardness, fracture toughness, and erosion showed the emulsion had no significant negative effects on enamel and dentin properties, confirming product safety.
The hydrophobic base of this peroxide-containing whitening emulsion is designed to be retained on teeth to promote contact time with the tooth surface, without trays or strips. The ability to target both kinds of stain is consistent with the emulsion group's significantly greater tooth color improvement when compared to that of the dentifrice group.
These study results support recommending emulsions for patients seeking a more convenient approach to tooth bleaching. Emulsion use was well-tolerated and considered an improvement over many patients' experience with "classic" tooth whitening products.10 The novel formulation has a hydrating base, thereby preventing dehydration, which is a primary contributor to bleaching-related sensitivity.21 In addition, the petrolatum base material has been demonstrated to prevent irritation from known irritants (eg, sodium hydroxide, lactic acid)25 and may be exerting a similar effect in this technology. Unlike some other whitening methods, the emulsion is applied in a single step and does not require product removal. The product's ease of application, efficacy, and excellent tolerability profile are appealing attributes to consumers.
Conclusion
A 2.5% hydrogen peroxidewhitening emulsion produced a significant whitening effect with excellent tolerability. Both b* (yellowness) and L* (lightness) were significantly improved relative to baseline values. Color substantially improved in 14 applications and continued to improve during the 21-day study period. The effect was consistently superior to that of a marketed whitening dentifrice. Whitening emulsions offer convenient, effective, well-tolerated tooth whitening benefit.
ACKNOWLEDGMENT
The authors thank Yamei Chen, Gang Wu, Xin Cheng, and Ruirui Zeng for assistance with study execution and Marisa DeNoble Loeffler, MS, for medical writing assistance in the preparation of this manuscript.
DISCLOSURE
This study, along with medical writing assistance, was funded by The Procter & Gamble Company. Authors NG and MKA are employees of The Procter & Gamble Company, and JL was an employee of Procter & Gamble Technology (Beijing) Co., Ltd. at the time the research was conducted. TH and HZ have no financial interest in the company whose materials are included in this article.
ABOUT THE AUTHORS
Tiezhou Hou, PhD
Professor, Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi'an Jiaotong University, Xi'an, Shaanxi, China; Professor, Clinical Research Center of Shaanxi Province for Dental and Maxillofacial Disease, College of Stomatology, Xi'an Jiaotong University; Professor, Department of Endodontics, Stomatological Hospital, College of Medicine, Xi'an Jiaotong University
Hongyan Zheng, MA
Vice General Manager, Beijing Sino German Union Cosmetic Institute Co., Ltd., Beijing, China
Nataliya Gurich, PhD
Clinical Scientist, The Procter & Gamble Company, Mason, Ohio, USA
Mary Kay Anastasia, BA
Senior Statistician, The Procter & Gamble Company, Mason, Ohio, USA
Jiahui Li, BS
Clinical Trial Manager, Procter & Gamble Technology (Beijing) Co., Ltd., Beijing, China
REFERENCES
1. Tin-Oo MM, Saddki N, Hassan N. Factors influencing patient satisfaction with dental appearance and treatments they desire to improve aesthetics. BMC Oral Health. 2011;11:6.
2. Van der Geld P, Oosterveld P, Van Heck G, Kuijpers-Jagtman AM. Smile attractiveness. Self-perception and influence on personality. Angle Orthod. 2007;77(5):759-765.
3. Alkhatib MN, Holt R, Bedi R. Prevalence of self-assessed tooth discolouration in the United Kingdom. J Dent. 2004;32(7):561-566.
4. Al-Zarea BK. Satisfaction with appearance and the desired treatment to improve aesthetics. Int J Dent. 2013;2013:912368.
5. Joiner A, Luo W. Tooth colour and whiteness: a review. J Dent. 2017;67S:S3-S10.
6. Meireles SS, Goettems ML, Dantas RV, et al. Changes in oral health related quality of life after dental bleaching in a double-blind randomized clinical trial. J Dent. 2014;42(2):114-121.
7. Pavicic DK, Kolceg M, Lajnert V, et al. Changes in quality of life induced by tooth whitening are moderated by perfectionism: a randomized, double-blind, placebo-controlled trial. Int J Prosthodont. 2018;31(4):394-396.
8. Bersezio C, Martín J, Angel P, et al. Teeth whitening with 6% hydrogen peroxide and its impact on quality of life: 2 years of follow-up. Odontology. 2019;107(1):118-125.
9. Estay J, Angel P, Bersezio C, et al. The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health. 2020;20(1):255.
10. Carey CM. Tooth whitening: what we now know. J Evid Based Dent Pract. 2014;14 suppl:70-76.
11. Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200(7):371-376.
12. Li Y. Biological properties of peroxide-containing tooth whiteners. Food Chem Toxicol. 1996;34(9):887-904.
13. Matis BA, Cochran MA, Franco M, et al. Eight in-office tooth whitening systems evaluated in vivo: a pilot study. Oper Dent. 2007;32(4):322-327.
14. Sutton Place Dental Associates. Chapter 1: In-office teeth whitening. Sutton Place Dental Associates website. https://www.suttonpda.com/in-office-teeth-whitening/. Accessed May 19, 2022.
15. Mokhlis GR, Matis BA, Cochran MA, Eckert GJ. A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime use. J Am Dent Assoc. 2000;131(9):1269-1277.
16. Haywood VB. Nightguard vital bleaching: current concepts and research. J Am Dent Assoc. 1997;128 suppl:19S-25S.
17. Matis BA, Cochran MA, Eckert G. Review of the effectiveness of various tooth whitening systems. Oper Dent. 2009;34(2):230-235.
18. Karadas M, Duymus ZY. In vitro evaluation of the efficacy of different over-the-counter products on tooth whitening. Braz Dent J. 2015;26(4):373-377.
19. Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006;34(7):412-419.
20. Matis BA. Degradation of gel in tray whitening. Compend Contin Educ Dent. 2000;(28 suppl):S28-S35.
21. Kugel G, Ferreira S. The art and science of tooth whitening. J Mass Dent Soc. 2005;53(4):34-37.
22. Commission Internationale de L'Eclairage. Recommendations on uniform color spaces. Color difference equations. Psychometric color terms. Suppl 2 to CIE pub 15 (E-13.1)1971/(TC-1.3), Paris, France: Bureau Central de la CIE; 1978.
23. Alqahtani MQ. Tooth-bleaching procedures and their controversial effects: a literature review. Saudi Dent J. 2014;26(2):33-46.
24. Suszcynsky-Meister E, St. John S, Schneiderman E. In-vitro safety evaluation of a hydrogen peroxide whitening emulsion technology on enamel and dentin. Am J Dent. 2022; in press.
25. Wigger-Alberti W, Elsner P. Petrolatum prevents irritation in a human cumulative exposure model in vivo. Dermatology. 1997;194(3):247-250.