Current State of Bone Replacement Grafting Materials for Dental Implants
Robert A. Horowitz, DDS
Contemporary dental implant therapy has flourished in recent years thanks in large part to advances made in bone augmentation capabilities. Bone grafts, which are often crucial to the success of implant restorations, are used mainly in three instances: at the time of extraction for delayed implant placement, when an immediate-socket implant is placed, and for ridge augmentation either laterally or vertically. Many bone grafting options are available to the surgeon depending on the site to be treated and the requirements in the area. Bone grafts and barriers come from different sources and are seen by the body's cell types in different manners. This yields a variety of biological outcomes, some of which have been published while others are in the process of being studied.
History and Concerns
Early periodontal studies followed oral surgery research and evaluated autogenous bone grafts used in patients with periodontal bone loss and defects in extraction sockets.1,2 Because of risks of disease transmission when using fresh frozen marrow, harvesting issues, or due to other factors, periodontists and many dentists have mostly switched to alternative sources of bone replacement grafts. Handschel and co-authors in a systematic review stated that for early implant placement in a grafted site the use or addition of autogenous bone is advantageous; however, they showed a decrease in total bone volume in sites grafted with autogenous bone over time.3 Other studies examining long-term bone volume in sinus grafting have shown significant decrease in volume with autogenous bone compared to other graft materials.4
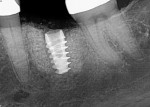
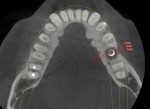
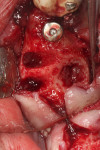
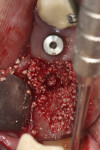
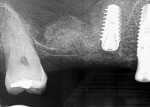
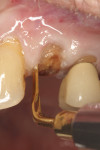
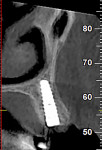
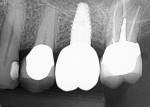
Numerous publications have covered the use of anorganic bovine bone mineral (ABBM), such as Bio-Oss® (Geistlich Pharma North America, geistlich-na.com), for ridge augmentation. Compared to autogenous block grafts, there is no chance of morbidity in the donor site. Also, more bone formation was noted when ABBM was combined with a high percentage of autogenous bone from the anterior mandible and placed under a titanium mesh for an anterior maxillary ridge augmentation.5 However, closely examined histologic specimens showed no osteoclastic activity and no resorption of the xenograft particles. Also, four of the seven meshes became exposed during healing.5 Any graft that is not resorbed and not either replaced by bone or surrounded by vital bone may potentially interfere with osseointegration. An example of this is shown in Figure 1 and Figure 2 in a patient after immediate-socket implant placement with an unknown graft by another surgeon. The periapical x-ray (Figure 1) shows crestal bone loss on the distal aspect with questionable bone formation on the mesial of the implant adjacent to the graft. On CBCT evaluation (Figure 2), a trough around the shoulder of the implant is evident.
Histologically, results were not as promising in sinus grafts or extraction sockets when ABBM was compared to mineralized cancellous allograft. In a bilaterally controlled sinus graft study, almost two times as much vital bone was found when allograft was inserted.6 In both conventional and simulated extraction sockets, similar histologic results were obtained with ABBM. In 9 months in humans, Tal's group found only 16% vital bone at the crest7 and deemed the bone graft to be nonresorbable.8 In the same relative timeframe in dogs (3 months), Artzi et al showed histologically that the graft particles almost completely encased in connective tissue in the central portion of the created defects.9 In humans, when a radiograph is taken in a buccolingual direction, all that is visible is the radiopaque graft material. If any radiopaque graft material is not resorbed, the clinician cannot determine when there is an increase in vital bone percentage in the site. This could be why some authors have advised that ABBM may not be an ideal graft material to place in extraction sockets.2
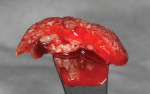
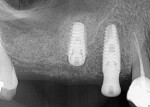
Alloplastic materials have been incorporated into sinus augmentation and extraction socket therapy, either alone or added to graft materials (Figure 3). Due to its physical and biologic activity, calcium sulfate can improve graft handling as well as angiogenesis10 and osteogenesis.11-13 Pure-phase beta-tricalcium phosphate (ß-TCP) has been studied in multiple indications from sockets to sinus grafting.14-17 Through particle shape and slow release of calcium, a long-term stimulation of bone formation occurs in the grafted site leading to actual bone formation. Because the graft particles are salts, they dissolve and are not dependent on osteoclastic resorption to be replaced by vital bone.
Novel Biomaterials and Growth Factors
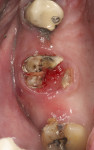
In patients or sites that are challenged, the use of bioactive materials may be beneficial to obtain improved physical and/or biologic results. In 2019, Dragonas and coworkers completed a literature search on various formulations and methods of fabrication of platelet-rich fibrin (PRF) and the effect on bone formation in different indications.18 Based on the literature, they could not conclude that there was an improvement in ridge preservation, augmentation (based on histomorphometric evidence), or bone quality in sinus augmentation. Miron et al reached similar conclusions in 2017.19 However, incorporation of PRF (Intra-Spin®, BioHorizons, biohorizons.com) does decrease washout of graft particles due to the ability to form "sticky bone" or "bone blocks" (Figure 4),20,21 provide anti-infective capabilities,22 and can enhance soft-tissue closure over the site.23
A recent retrospective case series of molar extraction sockets grafted with mineralized cancellous allograft demonstrated clinical and histologic success.24 Horowitz and Kurtzman achieved preservation of alveolar ridge width and an enhanced amount of keratinized tissue after grafting and barrier placement. Using atraumatic extraction techniques,25 the authors were able to preserve maximal blood supply and minimize damage to the facial plates of bone. Following available literature on techniques, grafts, and barriers26 led to appropriate choices of materials and instrumentation in their cases. The crestal bone and soft tissue have held up well over time. The vital bone percentage where analyzed was 47% to 70%,24 significantly higher than found with many other classes of bone replacement graft materials.
Clinicians and patients often prefer to avoid the use of autogenous bone harvesting for the treatment of deficient alveolar ridges. When ABBM is combined with recombinant human platelet-derived growth factor-BB (rhPDGF-BB), the literature has demonstrated turnover of ABBM, vital bone formation, and successful implant placement in dogs27 and humans.28,29 The technique in humans as described by Lee utilizes a tunnel approach with a remote vertical incision to deliver graft to the deficient site.29 Incorporating this growth factor when hydrating the bone replacement graft mixture enables recruitment and differentiation of mesenchymal stem cells from the elevated but not incised periosteum.30 Circumventing the use of an apical incision avoids damaging blood supply, eliminates chances of nerve injury,31 and decreases swelling and discomfort.
Biphasic calcium sulfate maintains its shape and can set on insertion in a socket.32 Its use can help preserve alveolar volume, enhance keratinized tissue, and enable vital bone formation in sockets. New research on a formulation with added hydroxyapatite has shown promise in alveolar ridge augmentation.33 Silica calcium phosphate nanocomposite is a bioactive TCP with sodium and silica ions. It is fully resorbable and has been shown clinically to preserve socket dimensions34 and upregulate cellular markers of bone formation.35 Unlike any other graft material studied so far, this material also has been shown to inhibit osteoclastic activity.36
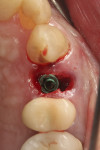
The patient shown in Figure 5 presented with pain in the maxillary right posterior. Flap elevation was performed to enable implant placement in site No. 4 at the time of extraction and socket grafting of No. 3 (Figure 6). The graft mixture used was bioactive silica-calcium phosphate composite prepared with leukocyte-PRF (L-PRF) block (as shown in Figure 3). Re-entry was performed 9 months later for implant placement in site No. 3 and crestal lifts in site Nos. 2 and 3 due to the patient's schedule. The same graft mixture that was used at the time of socket grafting was used for crestal sinus augmentation in both sites (Figure 7). An implant was inserted only in the first molar site. The periapical radiograph showed well-contained graft materials (Figure 8), attributed to the use of both the L-PRF block and a minimally invasive crestal elevation technique (Versah®, versah.com).37 At the 1-month radiographic evaluation the grafts appeared to be maturing and increasing in volume (Figure 9), unlike other graft materials that have been reported to lose volume in this area.
Autologous Dentin
An alternative method to harvesting autogenous bone is the utilization of autologous dentin. A tooth requiring extraction that has not been treated endodontically can be processed through a proprietary technique using a dentin grinding unit (Smart Dentin Grinder, KometaBio, kometabio.com). Using the dentin grinder, the surgeon obtains a sterile combination of small and large graft particles. These particles contain bone morphogenetic protein38-40 and other growth factors that stimulate healing in the recipient site. Clinically, this graft has been shown in animals to be superior to autogenous graft for bone volume formation.41 There was more expression of genes specific for bone formation revealing that this process was stimulated at a cellular level. This has been corroborated with an extraction socket study in humans by Pohl et al.42 Their results showed approximately a 1% loss in ridge width when a dentin graft mixed with PRF was placed at the time of atraumatic extraction and covered with either a collagen sponge or PRF barrier. This could be due in part to the stimulation and conversion of M1 to M2 macrophages.43
One indication where this autologous dentin material may be better biologically than what is reported in the literature is in immediate socket implants. The best esthetic results currently published are with a protocol involving ABBM in the gap, an immediate provisional restoration, and a connective tissue (or other graft) on the facial surface of the buccal plate.44,45 The procedural steps are highly demanding for a team of specialists, let alone an individual practitioner, requiring considerable experience and a high skill level to achieve the precision needed. Despite the outstanding clinical results, there are some biological concerns with the Levine et al protocol.44 As discussed earlier, a "slow-replacing" graft placed in the gap between the implant and buccal plate of bone may not resorb. Human and animal studies have shown these grafts can be encapsulated in connective tissue. In an at-risk patient or one who is not well-maintained, this could lead to the development of inflammation and peri-implant disease. Without significant amounts of vital bone around the graft particles and a blood supply to bring in the body's innate defense mechanisms, this inflammatory process could spread. Placing this type of graft on the palatal side of a thin buccal plate46,47 combined with a soft-tissue graft on the facial could restrict or eliminate the blood supply to the entire facial plate of bone. This could hasten osteoclastic destruction and buccal collapse vertically and horizontally. Where patients may be at risk for damaging immediate provisionals or have questionable home care, or where the dental team may be unable to execute restorative procedures at the desired optimal level, the incorporation of bioactive materials may be advisable.
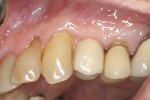
A 97½-year-old patient presented with a hopeless tooth No. 12 and was deemed a less-than-ideal candidate for immediate provisionalization. The tooth, which was fractured to the gingival margin, was extracted atraumatically25 with the use of piezosurgery (Mectron, dental.mectron.us) to minimize trauma to the bone and gingiva and decrease disruption of the blood supply (Figure 10). After placement of a dental implant in the palatal portion of the socket (Figure 11), the gap was grafted with partially demineralized ground dentin (KometaBio). The graft was fabricated into an L-PRF block and covered with an amnion-chorion barrier (BioXclude®, Snoasis Medical, snoasismedical.com), which has been shown to be anti-infective and contain growth factors and can be left in an exposed manner.48-50 A CBCT taken at the time of uncovering showed excellent bone-to-implant contact visually, alveolar width preservation, and graft consolidation with a radio-opacity identical to that of the surrounding bone (Figure 12). The soft-tissue profile 1 year after the implant was prosthetically loaded is shown in Figure 13. Presently, the patient is 102½ years old, and 5 years post-surgery the restoration looks the same or better.
Minimal Invasiveness
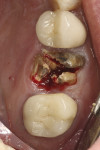
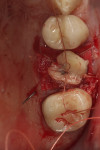
A current trend toward minimally invasive surgery is being aided by bioactive materials. As an example, a case is provided in Figure 14 through Figure 19. After a failed crown and cast post were removed, tooth No. 14 was sectioned to enable flapless, atraumatic extraction (Figure 14). The roots were removed and the sockets debrided and grafted with an L-PRF block of mineralized cancellous allograft (MinerOss®, BioHorizons). The bone graft was placed to ideal height and contour and covered with a collagen barrier (MemLok® Resorbable Collagen Matrix, BioHorizons) hydrated in the liquid expressed from the formation of L-PRF barriers. A plug of dense L-PRF was placed over the barrier (Figure 15) to help keep the patient from chewing and brushing in the area so as not to disturb early healing.
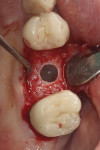
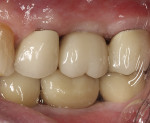
At 6 months, the site was re-entered with a full-thickness flap revealing full height and width preservation. A crestal sinus elevation was performed using the Versah drilling system (Figure 16),37,51 which is designed to increase bone-to-implant contact around the implant.52 The sinus membrane was elevated with the same graft mixture as placed in the initial socket defect. An implant (Tapered Pro, BioHorizons) was inserted into the osteotomy, a healing abutment was placed, and some of the remaining graft material was placed around it to support the papillae. The area was sutured and allowed to heal for 4 months prior to restoration. Clinically, the bone, keratinized tissue volumes, and size and shape of tooth No. 14 blended well with the adjacent teeth (Figure 17). Radiographic analysis showed excellent bone volume retention and remodeling in all areas it was placed (Figure 18). Histologic analysis showed a combination of new bone with osteoid and more mature bone (Figure 19). The core was 63% bone by volume, all vital in an area that has been reported as the weakest bone in the jaw and associated with high implant failure rates.53
Conclusion
Numerous biomaterials are available to dental surgeons. No one material will satisfy all of the requirements for all sites in all patients. For sinus grafting, while some materials have shown good clinical success, the surgeon may choose a different graft backed by literature and biology, depending on available residual native bone height or other factors where more vital bone could result.6,54-57 The same is true for extraction socket therapy, immediate socket implants, and ridge augmentation. The physical and physiologic conditions of the patient as well as the occlusal and biologic demands of the situation are crucial considerations for determining the most appropriate techniques, biomaterials, and growth enhancers to form or reform the ideal biologic complex for the recipient site. With an optimal mixture of vital bone, soft tissue, and vascularity, regenerated and restored biologic complexes around teeth or implants that are appropriately maintained should last the patient for many years.
DISCLOSURE
The author receives grant/research support, honoraria, and/or material support from the following companies cited in this article: Geistlich, KometaBio, Snoasis, and BioHorizons.
About the Author
Robert A. Horowitz, DDS
Adjunct Clinical Assistant Professor, Departments of Oral and Maxillofacial Surgery and Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York
References
1. Hiatt WH, Schallhorn RG. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J Periodontol. 1973;44(4):194-208.
2. Becker W, Clokie C, Sennerby L, et al. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998;69(4):414-421.
3. Handschel J, Simonowska M, Naujoks C, et al. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009;5:12. doi:10.1186/1746-160X-5-12.
4. Xavier SP, de Santana Santos T, Sehn FP, et al. Maxillary sinus grafting with fresh frozen allograft versus bovine bone mineral: a tomographic and histological study. J Craniomaxillofac Surg. 2016;44(6):708-714.
5. Proussaefs P, Lozada J, Kleinman A, et al. The use of titanium mesh in conjunction with autogenous bone graft and inorganic bovine bone mineral (Bio-Oss) for localized alveolar ridge augmentation: a human study. Int J Periodontics Restorative Dent. 2003;23(2):185-195.
6. Froum SJ, Wallace SS, Elian N, et al. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26(6):543-551.
7. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000;71(6):1015-1023.
8. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J Periodontol. 2001;72(2):152-159.
9. Artzi Z, Weinreb M, Givol N, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofacial Implants. 2004;19(3):357-368.
10. Strocchi R, Orsini G, Iezzi G, et al. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. J Oral Implantol. 2002;28(6):273-278.
11. Kim CK, Kim HY, Chai JK, et al. Effect of a calcium sulfate implant with calcium sulfate barrier on periodontal healing in 3‐wall intrabony defects in dogs. J Periodontol. 1998;69(9):982-988.
12. Quarles LD, Hartle JE, Siddhanti SR, et al. A distinct cation‐sensing mechanism in MC3T3‐E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res. 1997;12(3):393-402.
13. Pi M, Quarles LD. Osteoblast calcium‐sensing receptor has characteristics of ANF/7TM receptors. J Cell Biochem. 2005;95(6):1081-1092.
14. Horowitz RA, Mazor Z, Miller RJ, et al. Clinical evaluation alveolar ridge preservation with a beta-tricalcium phosphate socket graft. Compend Contin Educ Dent. 2009;30(9):588-590.
15. Horowitz RA, Mazor Z, Foitzik C, et al. β-tricalcium phosphate as bone substitute material: properties and clinical applications. J Osseointegration. 2010;2(2):61-68.
16. Plenk H Jr, Lederer J. Histomorphology of bone regeneration after sinus floor elevation with two types of TCP granulate - a case report. Zeitschrift für Orale Implantologie. 2005;32-38.
17. Knabe C, Koch C, Rack A, Stiller M. Effect of beta-tricalcium phosphate particles with varying porosity on osteogenesis after sinus floor augmentation in humans. Biomaterials. 2008;29(14):2249-2258.
18. Dragonas P, Katsaros T, Avila-Ortiz G, et al. Effects of leukocyte-platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: a systematic review. Int J Oral Maxillofac Surg. 2019;48(2):250-262.
19. Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913-1927.
20. Soni R, Priya A, Yadav H, et al. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl J Maxillofac Surg. 2019;10(1):98-101.
21. Upadhayaya V, Arora A, Goyal A. Bioactive platelet aggregates: Prp, Prgf, Prf, Cgf and sticky bone. IOSR J Dent Med Sci. 2017;16(5):5-11.
22. Haddadi P, Khorshidi H, Raoofi S, et al. Comparative evaluation of conventional and nanosilver-containing leucocyte and platelet-rich fibrin/biomaterial in the anti-biofilm formation of standard species of Candida and Streptococcus. Jundishapur Journal of Microbiology. 2018;11(8):1-6. doi:10.5812/jjm.68423.
23. Bilginaylar K. The use of platelet-rich fibrin for immediate closure of acute oroantral communications: an alternative approach. J Oral Maxillofac Surg. 2018;76(2):278-286.
24. Horowitz RA, Kurtzman GM. Socket preparation for delayed implant placement using a mineralized cancellous allograft. Compend Contin Educ Dent. 2021;42(4):f1-f4.
25. Horowitz RA, Mazor Z. Atraumatic extraction: advantages and implementation. Inside Dentistry.2010:6(7):68-74.
26. Horowitz R, Holtzclaw D, Rosen PS. A review on alveolar ridge preservation following tooth extraction. J Evid Based Dent Pract. 2012;12(3 suppl):149-160.
27. Simion M, Rocchietta I, Kim D, et al. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26(5):415-423.
28. Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007;27(2):109-115.
29. Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): a new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 2017;37(2):165-173.
30. Dhar K, Dhar G, Majumder M, et al. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer. 2010;9:209. doi:10.1186/1476-4598-9-209.
31. Mercier P, Zeltser C, Cholewa J, Djokovic S. Long-term results of mandibular ridge augmentation by visor osteotomy with bone graft. J Oral Maxillofac Surg. 1987;45(12):997-1004.
32. Horowitz RA, Rohrer MD, Prasad HS, et al. Enhancing extraction socket therapy with a biphasic calcium sulfate. Compend Contin Educ Dent. 2012;33(6):420-428.
33. Yahav A, Kurtzman GM, Katzap M, et al. Bone regeneration: properties and clinical applications of biphasic calcium sulfate. Dent Clin North Am. 2020;64(2):453-472.
34. Adel-Khattab D, Afifi NS, Abu El Sadat SM, et al. Bone regeneration and graft material resorption in extraction sockets grafted with bioactive silica-calcium phosphate composite (SCPC) versus non-grafted sockets: clinical, radiographic, and histological findings. J Periodontal Implant Sci. 2020;50(6):418-434.
35. Altwaim S, Al-Kindi M, AlMuraikhi N, et al. Assessment of the effect of silica calcium phosphate nanocomposite on mesenchymal stromal cell differentiation and bone regeneration in critical size defect. Saudi Dental Journal. In press.
36. El‐Ghannam A, Nakamura M, Muguruza LB, et al. Inhibition of osteoclast activities by SCPC bioceramic promotes osteoblast‐mediated graft resorption and osteogenic differentiation. J Biomed Mater Res A. 2021;109(9):1714-1725.
37. Huwais S, Mazor Z, Ioannou AL, et al. A multicenter retrospective clinical study with up-to-5-year follow-up utilizing a method that enhances bone density and allows for transcrestal sinus augmentation through compaction grafting. Int J Oral Maxillofac Implants. 2018;33(6):1305-1311.
38. Bessho K, Tanaka N, Matsumoto J, et al. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991;70(3):171-175.
39. Kawai T, Urist MR. Bovine tooth-derived bone morphogenetic protein. J Dent Res.1989;68(6):1069-1074.
40. Urist MR, Mizutani H, Conover MA, et al. Dentin, bone, and osteosarcoma tissue bone morphogenetic proteins. Prog Clin Biol Res. 1982;101:61-81.
41. Nampo T, Watahiki J, Enomoto A, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81(9):1264-1272.
42. Pohl S, Binderman I, Tomac J. Maintenance of alveolar ridge dimensions utilizing an extracted tooth dentin particulate autograft and platelet-rich fibrin: a retrospective radiographic cone-beam computed tomography study. Materials (Basel). 2020;13(5):1083.
43. Binderman I, Halperin‐Sternfeld M, Netanely E, et al. Biomaterials selection - cellular interactions during regenerative and remodeling phases. In: Artzi Z, ed. Bone Augmentation by Anatomical Region: Techniques and Decision‐Making. Wiley-Blackwell; 2020:chap 15:43-60.
44. Levine RA, Ganeles J, Gonzaga L, et al. 10 keys for successful esthetic-zone single immediate implants. Compend Contin Educ Dent. 2017;38(4):248-260.
45. Chu SJ, Salama MA, Garber DA, et al. Flapless postextraction socket implant placement, Part 2: the effects of bone grafting and provisional restoration on peri-implant soft tissue height and thickness - a retrospective study. Int J Periodontics Restorative Dent. 2015;35(6):803-809.
46. Huynh‐Ba G, Pjetursson BE, Sanz M, et al. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res. 2010;21(1):37-42.
47. Januário AL, Duarte WR, Barriviera M, et al. Dimension of the facial bone wall in the anterior maxilla: a cone‐beam computed tomography study. Clin Oral Implants Res. 2011:22(10):1168-1171.
48. Palanker ND, Lee CT, Weltman RL, et al. Antimicrobial efficacy assessment of human derived composite amnion-chorion membrane. Sci Rep. 2019;9(1):15600. doi:10.1038/s41598-019-52150-4.
49. Ashraf H, Font K, Powell C, Schurr M. Antimicrobial activity of an amnion-chorion membrane to oral microbes. Int J Dent. 2019;2019:1269534.
50. Holtzclaw D, Toscano N. Amnion-chorion allograft barrier: indications and techniques update. J Implant Adv Clin Dent. 2012;4(2):25-38.
51. Pai UY. Indirect sinus lift of atrophic posterior maxilla using osseodensification: a case report. J Indian Prosthodont Soc. 2018;18(suppl 2):S108.
52. Trisi P, Berardini M, Falco A, Podaliri Vulpiani M. New osseodensification implant site preparation method to increase bone density in low-density bone: in vivo evaluation in sheep. Implant Dent. 2016;25(1):24-31.
53. Jaffin RA, Berman CL. The excessive loss of Branemark fixtures in type IV bone: a 5-year analysis. J Periodontol.1991;62(1):2-4.
54. Galindo‐Moreno P, Moreno‐Riestra I, Ávila G, et al. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin Oral Implants Res. 2010;21(1):122-128.
55. Stumbras A, Krukis MM, Januzis G, Juodzbalys G. Regenerative bone potential after sinus floor elevation using various bone graft materials: a systematic review. Quintessence Int. 2019;50(7):548-558.
56. Danesh‐Sani SA, Engebretson SP, Janal MN. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta‐analysis. J Periodontal Res. 2017;52(3):301-312.
57. Velasco‐Ortega E, Valente NA, Iezzi G, et al. Maxillary sinus augmentation with three different biomaterials: histological, histomorphometric, clinical, and patient‐reported outcomes from a randomized controlled trial. Clin Implant Dent Relat Res. 2021;23(1):86-95.