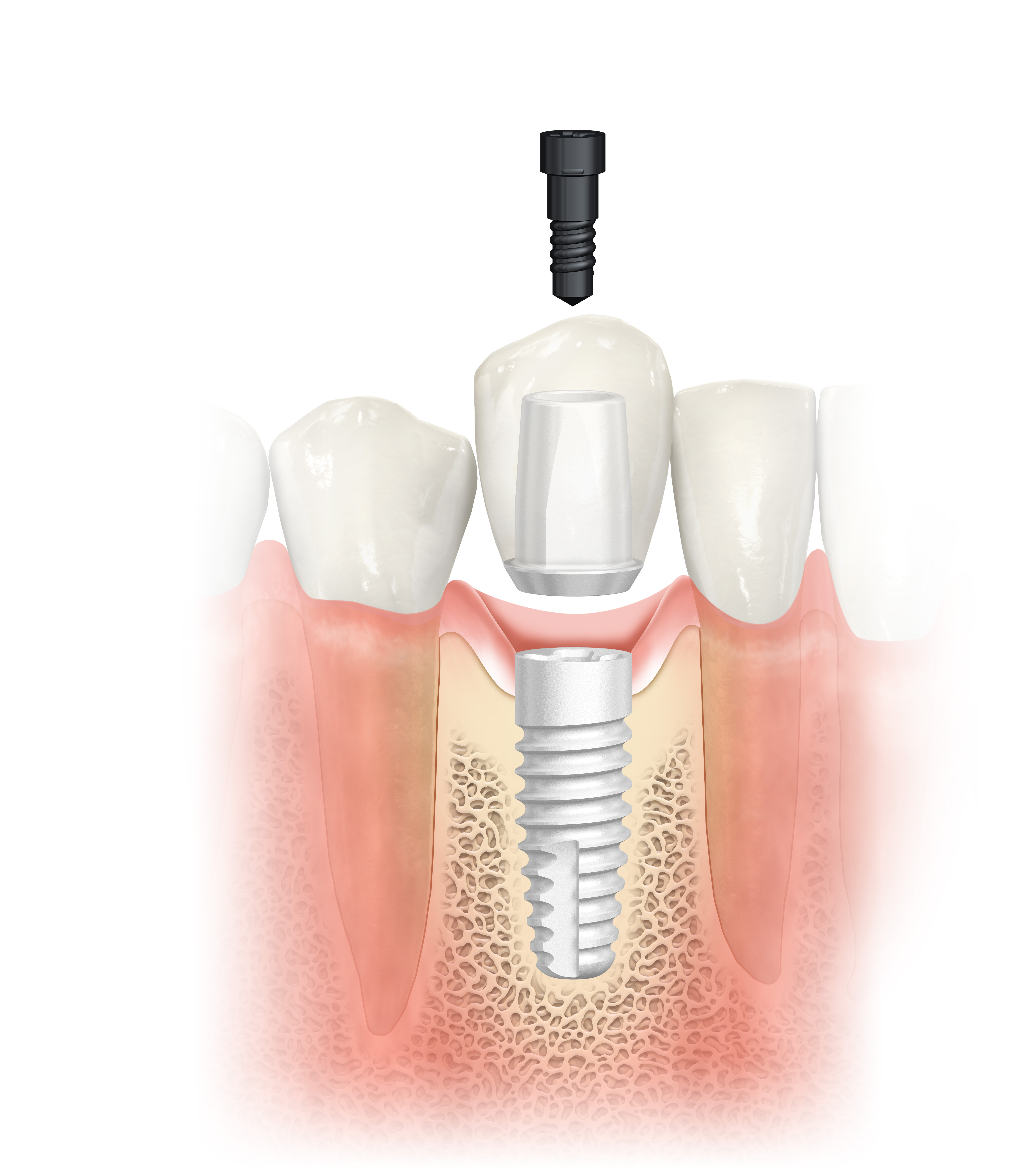
An alternative to titanium implants, the 100% metal-free NobelPearl was designed for excellent soft-tissue attachment and low inflammatory response1. Its zirconia material especially benefits patients with a thin mucosal biotype.2 Studies have shown that microcirculatory dynamics in peri-implant mucosa around zirconia are comparable with those around natural teeth.3 Furthermore, it has demonstrated low affinity to plaque.1,4,5
NobelPearl offers greater restorative flexibility compared with one-piece or cemented ceramic implants, owing to the two-piece, reversible cement-free internal connection. The Inter-X internal connection is specifically designed for ceramic implants. While the very strong ATZ ceramic absorbs compressive forces, the VICARBO® screw made of carbon fiber reinforced PEEK withstands tensile forces thanks to its continuous longitudinal carbon fibers. Risks of excess cement during intraoral cementation, often associated with soft-tissue inflammation and the development of peri-implant mucositis and peri-implantitis, can be avoided.6
Combined with the tapered drill protocol, the thread design and tapered implant shape of NobelPearl have been designed to achieve high primary stability. Osseointegration is achieved with help of the hydrophilic sand-blasted and acid-etched ZERAFIL™ surface as well as the partially machined collar.7 As no sintering or finishing takes place during the final shaping of the implant, a high level of dimensional precision and accuracy can be achieved.
The continuous development of ceramic implants by Nobel Biocare’s manufacturing partner, Dentalpoint AG, has seen an increase in survival rate over time. With NobelPearl, dental professionals now have everything they need for a successful start in ceramic implantology. It is available for a broad range of indications, from single to multiple unit, and follows established workflows for two-piece implants. It will also be integrated into Nobel Biocare’s digital workflow that includes treatment planning with the NobelClinician Software and guided implant surgery with NobelGuide pilot drilling. Additionally, clinicians will be able to offer patients the NobelPearl Ceramic Base CAD/CAM solution using DTX Studio design software later this year. Hans Geiselhöringer, President Nobel Biocare, said: “The demand by patients for ceramic implants has been steadily growing. With NobelPearl, we are proud to finally bring the first completely metal-free screw-retained two-piece implant system to the market. The result of many years of active research in ceramic dental implant technology, it adds an exciting full-ceramic metal-free dental implant option to Nobel Biocare’s leading range of titanium dental implants with the clinically proven TiUnite surface.”
More information about NobelPearl is available at nobelbiocare.com/pearl.
References
1. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where we are now, and where are we heading? Periodontol 2000 2017; 73(1):241-258
2. Cosgarea R, Gasparik C, Dudea D, et al. Peri-implant soft tissue colour around titanium and zirconia abutments: a prospective
randomized controlled clinical study. Clin Oral Implants Res 2015;26(5):537-544
3. Kajiwara N, Masaki C, Mukaibo T, et al. Soft tissue biological response to zirconia and metal implant abutments compared with
natural tooth: microcirculation monitoring as a novel bioindicator. Implant Dent 2015;24(1):37-41
4. Scarano A, Piattelli M, Caputi S, et al. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo
human study. J Periodontol 2004;75(2):292–296
5. Rimondini L, Cerroni L, Carrassi A, et al. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J
OralMaxillofac Implants 2002;17(6):793–798
6. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic
study. J Periodontol 2009;80(9):1388-1392
7. Chappuis V, Cavusoglu Y, Gruber R, et al. Osseointegration of zirconia in the presence of multinucleated giant cells. Clin Implant
Dent Relat Res 2016;18(4):686–698
Nobel Biocare is a world leader in the field of innovative implant-based dental restorations. The company’s portfolio offers
solutions from single tooth to fully edentulous indications with dental implant systems (including key brands NobelActive®,
NobelParallel®, Brånemark System® and NobelReplace®), a comprehensive range of high-precision individualized prosthetics and
CAD/CAM systems (NobelProcera®), treatment planning and guided surgery solutions (NobelClinician® and NobelGuide®) and
biomaterials (creos™). Nobel Biocare supports its customers through all phases of professional development, offering world-class
training and education along with practice support and patient information materials. The company is headquartered in Zurich,
Switzerland. Production takes place at five sites located in the United States, Sweden, Japan and Israel. Products and services are
available in over 80 countries through subsidiaries and distributors.




