You must be signed in to read the rest of this article.
Registration on AEGIS Dental Network is free. Sign up today!
Forgot your password? Click Here!
Plaque Reduction Efficacy of an Oscillating-Rotating Power Brush with a Novel Brush Head Utilizing Angled Bristle Tufts
Abstract:
PURPOSE: To evaluate an oscillating-rotating power brush with a novel brush head utilizing angled bristle tufts versus a manual brush for plaque removal. METHODS: This was a single-center, randomized, open-label, examiner-blind, two-treatment, parallel-group study. Subjects brushed with their assigned toothbrush and a marketed dentifrice twice daily at home for 6 weeks. Plaque measurements were evaluated at baseline and week 6 using the Turesky Modified Quigley-Hein Plaque Index (TQHPI). Data was analyzed using the analysis of covariance (ANCOVA) with baseline as the covariate. RESULTS: Ninety-four subjects completed the study, with 46 in the manual group and 48 in the power group. The oscillating-rotating brush with the novel brush head demonstrated statistically significantly greater reductions in whole mouth and interproximal plaque measures compared to the manual toothbrush. The benefit for the oscillating-rotating brush over the manual control brush was 164.5% for whole mouth plaque and 167.4% for interproximal plaque (P < 0.001) measured 12 hours after brushing. Both brushes produced statistically significant reductions in plaque measures relative to baseline (P < 0.001 for both measures). There were no adverse events reported or observed for either brush. CONCLUSIONS: The oscillating-rotating brush with the novel brush head produced reductions in whole mouth and interproximal plaque more than twice that of the manual toothbrush.
Removal of plaque biofilm from the tooth surface is vital for prevention of oral conditions such as caries, gingivitis, and periodontitis. The most common method of oral hygiene is manual toothbrushing. Today’s market offers a vast array of toothbrush designs for consumers to choose from and, likewise, professionals must make an educated recommendation based on evidence available in the literature.
The initial design of the modern manual toothbrush was a flat-trim, multi-tufted, end-rounded nylon filament brush and was used in various forms for many years with minor design modifications.1 In an effort to improve the ability of a toothbrush to reach the interproximal areas and be more effective in controlling plaque levels, manufacturers began to develop brushes with various types of bristle profiles. For example, three common designs include flat-trim, multi-level (with a raised toe or rows of bristle having different lengths), and, more recently, brushes with angled bristles.
Slot et al recently looked at the efficacy of manual toothbrushing with respect to toothbrush design in a systematic review of 212 brushing exercises in 10,806 participants.2 Findings demonstrated that angled bristle tuft arrangements have made a significant contribution to enhancing plaque removal over flat-trim and multi-level designs. Since its creation, the CrossAction® toothbrush (Oral-B®, Procter & Gamble, www.dentalcare.com) with 16-degree angled bristles has been proven to maximize plaque removal, specifically in hard-to-reach areas, relative to various manual and power toothbrush controls.3-5
Recently, a new oscillating-rotating power brush head was developed with 16-degree angled bristle tufts, based on the CrossAction manual toothbrush design. The novel brush head, Oral-B® CrossAction, features an outer ring of bristles angled at +16 degrees for oscillation’s forward direction, while the inner ring of bristles has an angle of -16 degrees for the backward direction. The inner part of the brush head has only minor movement. The brush head was designed to optimize shear force transmission and increase penetration of interproximal areas.
Purpose
The purpose of this study was to compare the effectiveness of the novel brush head with angled bristles to a standard manual flat-trim brush for plaque removal over a 6-week period.
Methods
This was a single-center, randomized, two-treatment, examiner-blind, parallel-group study. Clinical evaluations were completed at baseline and week 6. The study protocol was approved by the Institutional Review Board (U.S. IRB, Miami, Fla.). One-hundred subjects, 50 per group, were recruited and asked to sign a written informed consent prior to their participation in the study. At the baseline visit, subject medical history and demographics were obtained and those subjects that satisfied the study inclusion/exclusion criteria were enrolled.
To qualify for the study, subjects were required to have a whole mouth mean baseline plaque score ≥ 1.75 using the Turesky Modified Quigley Hein Plaque Index (TQHPI).6 In addition, the subjects had to be at least 18 years of age, in good general health, have a minimum of 16 natural teeth with facial and lingual valid surfaces, and have been previously screened as a consistent manual brush user. Subjects were not allowed to participate in any other oral care study or use non-study oral hygiene products for the duration of this study and they would agree to delay any elective dentistry, including a prophylaxis, until study completion.
Subjects were asked to refrain from brushing their teeth and performing any other oral hygiene procedures for 12 hours prior to each visit and refrain from eating, chewing gum, drinking, and tobacco use for 4 hours prior to each visit, except for small sips of water up until 45 minutes prior to each visit. Subjects were also excluded if there was evidence of any disease or condition that may interfere with study procedures or if antibiotics or chlorhexidine mouth rinse had been used within 2 weeks prior to the start of the study.
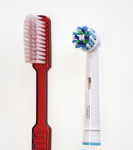
The study test products (Figure 1) included:
• the novel brush head with angled bristles on an oscillating-rotating handle (Oral-B® Professional Care 1000, sold as Oral-B® Professional Care 600 in some regions, with Oral-B® CrossAction brush head, D16u/EB50, Procter & Gamble).
• the control brush, ADA reference, manual, soft toothbrush (American Dental Association, www.ada.org).
Each brush was used with standard dentifrice containing 0.243% sodium fluoride (Crest® Cavity Protection, Procter & Gamble).
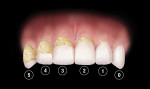
At the baseline visit, eligible subjects were given an oral soft-tissue examination and then asked to swish with 2.5 ml FD&C red #28 disclosing solution for 10 seconds followed by a water rinse for 10 seconds to stain the plaque present on their teeth. A plaque assessment (TQHPI) (Figure 2) was conducted by an experienced examiner immediately following the disclosing solution rinse. The assessments were conducted as described by Klukowska et al.7
Subjects were then stratified based on gender, baseline whole mouth mean TQHPI scores (≤ 2.3 vs. > 2.3), and age (≤ 35 vs. > 35). The subjects were then randomly assigned to one of the two treatment groups: power or manual.
Each subject was supplied with their assigned toothbrush and a fluoride dentifrice in an area separate from the examination room to ensure the examiner was blinded to treatment assignment since the brushes were used open label. Under supervision by a member of the research staff not associated with the clinical assessments, subjects then received oral hygiene and product usage instructions, and brushed in front of a mirror for on-site practice. Subjects in the power brush group were then instructed to brush for 2 minutes twice daily (according to manufacturer’s instructions) at home for 6 weeks. Subjects in the manual brush group were instructed to brush their teeth as they normally do. The on-site supervised brushing at the baseline visit was considered one of the subject’s twice-daily brushings.
Subjects were scheduled to return to the research center with their study product for their week 6 (± 3 days) assessment visit. They were reminded by phone call or email prior to this visit that they should refrain from brushing their teeth for 12 hours prior to their appointment time, and that for 4 hours before the visit they were to refrain from eating, chewing gum, drinking, or using tobacco products. Continuance criteria were assessed prior to examinations taking place. Once eligibility was determined, subjects received an oral soft-tissue examination and TQHPI plaque assessments in the same manner as conducted during the baseline visit.
The safety assessment involved both the hard and soft tissues of the oral cavity. All non-serious voluntarily reported whole-body adverse events that were potentially product related, as well as all oral adverse events, were recorded. In addition, all serious adverse events were documented and reported appropriately.
Power analysis was conducted with a = 0.05, using a two-sided test and a sample size of 50 subjects per group using a variability estimate of whole mouth plaque reduction of 0.247; a sample size of 50 subjects per group should provide 90% power to detect a difference in TQHPI mean reduction scores as small as 0.162 units between treatments.
Age was compared for group differences using a two-sample t-test, and gender and race were compared using a Chi-Square test and Fisher’s Exact test, respectively. Statistical analysis for plaque efficacy was based on average whole mouth TQHPI change from baseline score (baseline minus week 6). The 6-week plaque reduction was analyzed for treatment differences using an analysis of covariance (ANCOVA) model with baseline whole mouth TQHPI score as the covariate. Similar analyses were conducted for interproximal scores. All treatment comparisons were two-sided with a significance level of a = 0.05.
Results
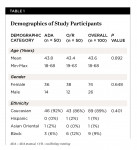
A total of 100 subjects were enrolled in the study and randomized to treatment, with 50 subjects in each treatment group. Ninety-four subjects completed the study, with 46 in the manual brush group and 48 in the oscillating-rotating brush group. Subjects were 43.6 years old on average, ranging from 18 to 68 years, and 74% of the subjects were female. Both groups were well-balanced (P > 0.40) on age, gender, and ethnicity. See Table 1.
Subjects presented with ≥ 1.75 plaque accumulation at the baseline visit. The whole mouth plaque reductions were statistically significant from baseline for both brushes (P < 0.001). The oscillating-rotating power brush had a 164.5% higher whole mouth plaque reduction (P < 0.001) compared to the control manual brush after 6 weeks of brushing.
In addition, plaque measured at the interproximal areas of the teeth was statistically significantly reduced from baseline for both power and manual toothbrushes (P < 0.001). The oscillating-rotating power brush had a 167.4% greater interproximal plaque reduction (P < 0.001) compared to the control manual brush after 6 weeks of use. See Table 2.
No product-related adverse events or safety concerns were observed or reported in either group.
Discussion
These study results demonstrate significant plaque removal advantages for the new brush head for both whole mouth and interproximal, hard-to-reach areas. The oscillating-rotating brush had more than two times the whole mouth and interproximal plaque reduction 12 hours after brushing compared with a standard manual flat-trim brush after 6 weeks of use, demonstrating the efficacy of the 16-degree angled bristle configuration in the new brush head. To mimic real-life brushing behavior, subjects in the power group were instructed to brush following the manufacturer’s instructions (two minutes twice a day), and those in the manual group were instructed to brush in their customary manner twice a day.
In addition, this novel brush head on the oscillating-rotating handle has been shown to have efficacy advantages versus advanced sonic toothbrushes for plaque removal as well as gingivitis reduction. Klukowska et al conducted a clinical trial that demonstrated statistically superior reductions in plaque and gingivitis for the oscillating-rotating brush head utilizing angled bristles versus Sonicare DiamondClean (Philips Oral Healthcare, www.philipsoralhealthcare.com) after 6 weeks of brushing.8 The plaque removal advantages, based on the Rustogi-Modified Navy Plaque Index, were 22% for whole mouth plaque and 33% for interproximal plaque; gingivitis and gingival bleeding benefits ranged from 32% to 35%. A second independent study by Klukowska and colleagues also showed advantages for the novel oscillating-rotating brush head relative to Colgate® ProClinical® A1500 (Colgate-Palmolive Co., www.colgateprofessional.com), a sonic toothbrush with self-adjusting technology.9 The benefit for the oscillating-rotating brush over the sonic brush was 21.3% for gingivitis, 35.7% for gingival bleeding, 34.7% for number of bleeding sites, 17.4% for whole mouth plaque, and 21.2% for interproximal plaque.
Combining an effective power toothbrush handle with a brush head design that maximizes plaque removal, particularly interproximal plaque removal, is an advantage for both oral health providers and their patients to improve home care. According to a recent report by the Centers for Disease Control and Prevention, approximately half of Americans aged 30 or older, 64.7 million people, are reported to have advanced periodontal disease, ie, periodontitis.10 While a number of factors put patients at a higher risk for developing periodontal disease, such as age, smoking, genetics, low income, and health history, controlling dental plaque is an important step all patients should take to try to reduce their risk of the first stage of periodontal disease, gingivitis. It is understood that gingivitis can be prevented if there is adequate home care, so adopting new oral health products might be the key to lowering the percentage of persons inflicted with periodontal disease.
Clinical Implications
The consequences of inefficient plaque removal, specifically in the interproximal areas, are a concern for the population’s oral health. The advanced oscillating-rotating power toothbrush with the novel brush head utilizing angled bristle tufts can significantly reduce plaque levels versus a manual toothbrush, helping patients achieve better oral health.
DISCLOSURE
The study was supported by the Procter & Gamble Company. Drs. Klukowska and Grender and Ms. Conde are employees of the Procter & Gamble Company. Dr. Milleman and Ms. Milleman have no conflicts to disclose.
ABOUT THE AUTHORS
Malgorzata Klukowska, DDS, PhD
Principal Clinical Scientist, Procter & Gamble Company, Mason, Ohio
Julie M. Grender, PhD
Research Fellow Statistician, Procter & Gamble Company, Mason, Ohio
Erinn Conde, BS
Clinical Trial Manager, Procter & Gamble Company, Mason, Ohio
Kimberly R. Milleman, RDH, BSEd, MS
Director, Salus Research, Inc., Fort Wayne, Indiana
Jeffery L. Milleman, DDS, MPA
Director, Salus Research, Inc., Fort Wayne, Indiana
REFERENCES
1. Cugini M, Warren PR. The Oral-B CrossAction manual toothbrush: A 5-year literature review. J Can Dent Assoc. 2006;72(4):323.
2. Slot DE, Wiggelinkhuizen L, Rosema NA, Van der Weijden GA. The efficacy of manual toothbrushes following a brushing exercise: a systematic review. Int J Dent Hyg. 2012;10(3):187-197.
3. Sharma NC, Qaqish JG, Galustians HJ, et al. An advanced toothbrush with improved plaque removal efficacy. Am J Dent. 2000;13(spec no):15A-19A.
4. Cronin MJ, Dembling WZ, Low MA, et al. A comparative clinical investigation of a novel toothbrush designed to enhanced plaque removal efficacy. Am J Dent. 2000;13(spec no):21A-26A.
5. Sharma NC, Qaqish JG, Galustians HJ, et al. A 3-month comparative investigation of the safety and efficacy of a new toothbrush: results from two independent clinical studies. Am J Dent. 2000;13(spec no):
27A-32A.
6. Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41(1):41-43.
7. Klukowska M, Grender JM, Timm H. A single-brushing study to compare plaque removal efficacy of a new power brush to an ADA reference manual toothbrush. Am J Dent. 2012;25 spec no A(A):10A-13A.
8. Klukowska M, Grender JM, Conde E, et al. A 6-week clinical evaluation of the plaque and gingivitis efficacy of an oscillating-rotating power toothbrush with a novel brush head utilizing angled CrissCross® bristles versus a sonic toothbrush. J Clin Dent. 2014;25(2):6-12.
9. Klukowska M, Grender JM, Conde E, et al. A randomized clinical trial evaluating gingivitis and plaque reduction of an oscillating-rotating power brush with a new brush head utilizing angled bristle tufts versus a marketed sonic brush with self-adjusting technology. Am J Dent. In press.
10. Eke PI, Dye B, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):907-908.