You must be signed in to read the rest of this article.
Registration on AEGIS Dental Network is free. Sign up today!
Forgot your password? Click Here!
Endodontic Obturation Using a Bioceramic Sealer
Biologic advantages contribute to fast periapical healing
Warley Luciano Fonseca Tavares, DDS, PhD
The success of endodontic treatment and the rehabilitation of the periapical tissues are intimately related to proper root canal disinfection and the physicochemical and biologic properties of the endodontic sealers that are used.1,2 Throughout the years, clinicians have sought to find an ideal root canal sealer, and in the past, cements based on zinc oxide, eugenol, and resin were the most used materials.1,2
Formulated from Portland cement, mineral trioxide aggregate (MTA) emerged in the early 1990s as a promising material for treating cases involving perforation, resorption, and retrofilling. This was largely driven by its biocompatibility and ability to seal, even in the presence of moisture or blood. Later, new studies showed that in addition to these properties, MTA also exhibited bioactivity, and the material soon became the gold standard for solving complex cases.3-9
However, characteristics such as a sandy consistency and a lack of flow as well as the difficulty of insertion and removal, especially in curved canals,9 restricted the use of this material as a filling cement. In order to transcend these limitations, bioceramic sealers composed of silicates, which are particles that are similar to those found in MTA, have recently been developed. These materials exhibit the biologic advantages inherent to MTA; however, their physicochemical properties allow them to be more efficiently used in the filling of the root canal system.10 This article presents two cases involving the use of a bioceramic root canal sealer.
Case No. 1
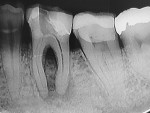
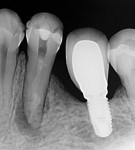
A 45-year-old man was referred for the endodontic treatment of his mandibular left first molar. Sensitivity tests were negative, but the patient presented with swelling and a sinus tract. The tooth was diagnosed as necrotic with chronic apical periodontitis. An extended interradicular and periapical lesion was also observed during the radiographic evaluation (Figure 1). At the first appointment, the patient was anesthetized using lidocaine with 1:100,000 epinephrine (Alphacaine, DFL Indústria e Comércio S/A), and the canals were instrumented with a series of rotary NiTi files (Bassi Logic™ Shaping & Glidepath Files, Bassi Endo) up to size 30/.05 and irrigated with a 2.5% sodium hypochlorite (NaOCl) solution. After a 17% ethylenediaminetetraacetic acid (EDTA) solution was applied for 60 seconds, the canals were filled with calcium hydroxide, and it was left in place for 15 days until the next appointment.
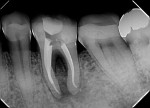
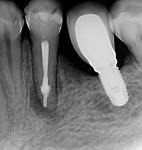
During the second session, the root canal dressing was removed, and the canals were irrigated again using the following protocol. First, 3 mL of 2.5% NaOCl solution was introduced and agitated for 60 seconds with a rotary plastic activating file (Bassi Clean™, Bassi Endo) at 20,000 rpm. Next, 1 mL of 17% EDTA was placed and agitated for 60 seconds at 20,000 rpm. And last, another 3 mL of 2.5% NaOCl solution was placed and agitated for 60 seconds at 20,000 rpm, followed by a final irrigation with 1 mL of saline solution. After the canals were properly dried with absorbent paper cones, the root canal obturation was performed with a bioceramic root canal sealer (BIO-C® Sealer, Angelus) and gutta-percha using the lateral condensation technique (Figure 2 and Figure 3). The tooth received a fiberglass post in the distal canal and was restored with composite resin. At the 6-month follow-up visit, the patient was asymptomatic, and complete healing of the periapical tissues was observed (Figure 4).
Case No. 2
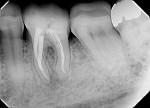
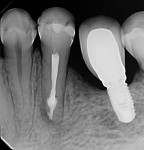
A 50-year-old woman was referred for the endodontic treatment of her mandibular left second bicuspid. The patient was suffering from pain and presented with swelling and a sinus tract. During the radiographic exam, the presence of root resorption and a periapical lesion could be observed (Figure 5). The canal was instrumented using a series of rotary NiTi files (Bassi Logic Shaping & Glidepath Files, Bassi Endo) up to size 50/.01 and then copiously irrigated with a 2.5 % NaOCl solution. Next, a 17% EDTA solution was used, and then the canal was filled with calcium hydroxide, which would remain in place as an intracanal medication for 15 days (Figure 6).
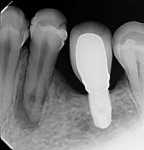
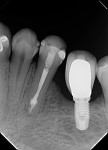
At the following appointment, the calcium hydroxide was removed, and the canal was irrigated using the same protocol that was used in case No. 1. First, 3 mL of 2.5% NaOCl solution was placed and agitated for 60 seconds at 20,000 rpm with the plastic activating file. Then, 1 mL of 17% EDTA solution was agitated for 60 seconds at 20,000 rpm, followed by 3 mL of 2.5% NaOCl solution that was agitated for 60 seconds at 20,000 rpm, and a final irrigation with 1 mL of saline solution. After irrigation, the canal was properly dried with absorbent paper cones. The tooth was then filled with the bioceramic root canal sealer (BIO-C® Sealer, Angelus) and gutta-percha using a lateral condensation technique associated with the use of the McSpadden condenser (ie, Tagger's hybrid technique) (Figure 7).11 The final restoration was performed with composite resin. At the 3-month (Figure 8) and 18-month (Figure 9) follow-up visits, healing of the periapical tissue was observed, and the patient was completely asymptomatic.
Conclusions
In the cases presented, the performance of proper root canal disinfection prior to the 3-dimensional filling of the root canals was paramount to the success of the procedures. The complete healing of the periapical tissues and elimination of postoperative pain or discomfort during the short follow-up period after each case (ie, 3 to 18 months) was remarkable. The bioceramic root canal sealer was shown to be a valuable material with considerable biologic properties that contributed to fast periapical healing.
About the Author
Warley Luciano Fonseca Tavares, DDS, PhD
Professor
Department of Restorative Dentistry
School of Dentistry
Federal University of Minas Gerais
Belo Horizonte, Brazil
References
1. de Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, et al. In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod.2003;29(2):95-99.
2. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377-390.
3. Rezende TM, Vargas DL, Cardoso FP, et al. Effect of mineral trioxide aggregate on cytokine production by peritoneal macrophages. Int Endod J. 2005;38(12):896-903.
4. Rezende TM, Vieira LQ, Cardoso FP, et al. The effect of mineral trioxide aggregate on phagocytic activity and production of reactive oxygen, nitrogen species and arginase activity by M1 and M2 macrophages. Int Endod J. 2007;40(8):603-611.
5. Liu S, Wang S, Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod. 2015;41(5):652-657.
6. Braga JM, Oliveira RR, de Castro Martins R, et al. Assessment of the cytotoxicity of a mineral trioxide aggregate-based sealer with respect to macrophage activity. Dent Traumatol. 2015;31(5):390-395.
7. Koch KA, Brave DG. Bioceramics, part I: the clinician's viewpoint. Dent Today. 2012;31(1):130-135.
8. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197-205.
9. Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35(6):777-790.
10. Borges RP, Sousa-Neto MD, Versiani MA, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45(5):419-428.
11. Tagger M. Use of thermo-mechanical compactors as an adjunct to lateral condensation. Quintessence Int Dent Dig. 1984;15(1):27-30.
For more information, contact:
angelus
https://www.angelusdental.com/
855-346-3682